The decision of a blastomere to become inner cell mass or trophectoderm relies on the integration of two types of information: position and polarity. Compaction and polarization play key roles in this first lineage decision, since they alter both the intra- and the intercellular organization of the embryo. In-depth studies of early embryogenesis using the mouse model have provided new insights into the molecular regulation of compaction, polarization and lineage specification. However, how these processes first emerge and influence subsequent molecular and cellular events remain open questions in the field. In this review, we summarize the chain of events that lead to the generation of the first two cell lineages, outlining how compaction and polarization can build cell identity. Such processes, despite running in parallel, are subjected to different regulatory pathways. Then, if under specific circumstances one of the regulatory pathways is affected, embryos may achieve compaction but may have severe problems to acquire full developmental potential.
La decisión de una blastómera embrionaria de formar parte de la masa celular interna o del trofoectodermo requiere de la integración de dos tipos de información: posición y polaridad. La compactación y la polarización son dos eventos clave en esta primera decisión de destino, ya que alteran la configuración intra- e intercelular del embrión. Estudios detallados de la embriogénesis temprana utilizando el modelo de ratón han arrojado luz a la regulación molecular de dichos eventos. Sin embargo, cómo cada uno de estos procesos emergen e influyen en los sucesivos acontecimientos moleculares y celulares continúa siendo una cuestión abierta. En esta revisión resumimos la cadena de eventos que conducen a la generación de los dos primeros linajes celulares, describiendo cómo compactación y polarización pueden modelar la identidad celular. A pesar de que ambos eventos transcurren de forma paralela, estos están sujetos a diferentes vías de regulación. Por ello, si en circunstancias específicas una de las vías de regulación se viera afectada, los embriones podrían mostrar signos de compactación, pero su potencial evolutivo estaría seriamente afectado.
During mammalian preimplantation development, the embryo undergoes a series of morphogenetic processes that lead to the formation of a complex structure called blastocyst. The mammalian blastocyst is originally composed of two distinct cell types: the trophectoderm (TE) and the inner cell mass (ICM); which emerge from a totipotent zygote after several rounds of proliferative cell divisions. The very first cell fate decision, which involves the activation of a specific lineage expression program according to the molecular and cellular microenvironment, rely on two preceding key events: compaction and polarization, since these influence the intra- and intercellular organization of the developing embryo (Mihajlovic and Bruce, 2017). Compaction is the process by which a loosely bound cluster of blastomeres spread their adhesion interfaces to form a tightly packed mass of cells called morula. Concomitantly, polarization triggers the asymmetrical allocation of certain subcellular components, resulting in molecularly heterogeneous cells that break the global embryo symmetry after a round of asymmetric division. Given the importance of these two events, embryos that fail to compact or polarize cannot reach the blastocyst stage, hence leading to developmental failure (Stephenson et al., 2010; Larue et al., 1994; Kim et al., 2017; Zhu et al., 2017).
The general scheme of early mammalian embryogenesis has long been known. However, the mechanisms that initiate the morphogenetic events leading to the first lineage bifurcation are only just beginning to be understood (Chazaud and Yamanaka, 2016). In-depth explorations of cell-fate specification mechanisms have been severely hampered by the extraordinary plastic and self-organizing features of early mammalian development: cells of the preimplantation embryo retain the potential to amend former fate choices in response to environmental changes until well after the symmetry breaking (Bedzhov et al., 2015). Moreover, owing to material scarcity and to ethical and legal constraints regarding research with human embryos, most of our knowledge of early mammalian development is based on mouse observations, and is thus biased by fundamental species-specific differences (Jedrusik, 2015).
The molecular characterization of the mechanisms that hide behind embryonic morphogenesis and patterning remains a major issue in the field of developmental biology. The general aim of this review is to assess the current knowledge on the molecular basis of the morphogenetic events leading to the first lineage bifurcation. To this end we will examine the evidence accumulated in the mouse model, raising still unanswered questions and eventually highlighting key differential aspects with regard to the human species.
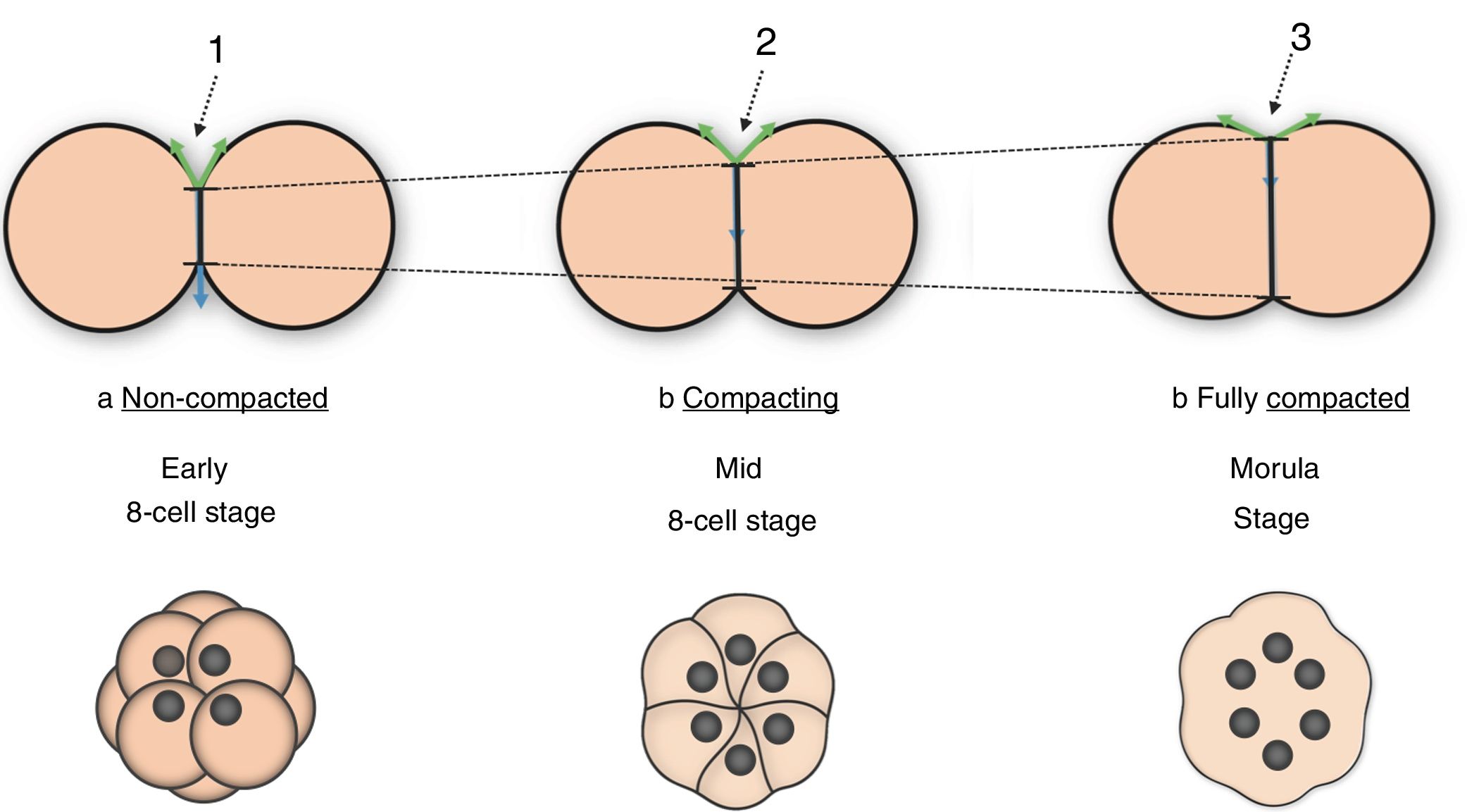
Cell adhesion changes drive embryo compaction at the 8-cell stageTissue deformation during embryogenesis is accomplished by continuous changes in the degree of cellular cohesion. Compaction is the morphogenetic process by which forming tissues compress, acquiring a tighter and more organized configuration within the embryo. In the mouse, embryo compaction is primarily initiated at the 8-cell stage. First, adjacent membranes come close to each other and cell contact interfaces spread. Then, flattened but still well-delineated membranes start to establish adhesive interactions, resulting in a hollow ball of cells whose membranes at the contact sites are smooth and no longer discernible (Fig. 1). The formation of this tight structure, called morula, is the first manifestation of the compaction process during mammalian embryo development (Cockburn and Rossant, 2010; Gardner, 1989; Alikani, 2005).
Cell–cell interface spreading during embryo compaction. (a) The non-compacted embryo can be easily recognized as a loose cluster of relatively spherical blastomeres, whose contact interfaces are small (black arrow 1). (b) Compaction initiation is marked by the spreading of the cell contact interfaces (black arrow 2). (c) The morula is the output of compaction, and shows no distinguishable cell boundaries and maximized contact interfaces (black arrow 3).
Intercellular communication at compaction is achieved by the formation of adherens junctions (AJs). These binding complexes consist of membrane-anchored Ca2+-dependent E-cadherin (E-cad) bound to intracellular β-catenin and α-catenin (Stephenson et al., 2010; De Vries et al., 2004). In the mouse embryo E-cad is maternally supplied and expressed de novo from the 2-cell stage (Vestweber et al., 1987). A number of studies have shown how interfering with the establishment of AJs can hamper compaction to varying extents. For instance, embryos deprived of maternal E-cad show delayed compaction at the 16-cell stage (De Vries et al., 2004). Knock-out embryos lacking zygotic E-cad (Larue et al., 1994) or α-catenin (Torres et al., 1997) can compact at the 8-cell stage thanks to their maternal reservoir, but they are preimplantation lethal as they are unable to form a proper TE epithelium. β-Catenin depletion does not compromise development until gastrulation, possibly thanks to maternal compensation or molecular redundancy with γ-catenin (Haegel et al., 1995). Embryos lacking both maternal and zygotic E-cad fail to compact at all, and they remain as a loose cluster of cells (Stephenson et al., 2010). Furthermore, Ca2+ depletion from the culture media causes morula decompaction (Pey et al., 1998).
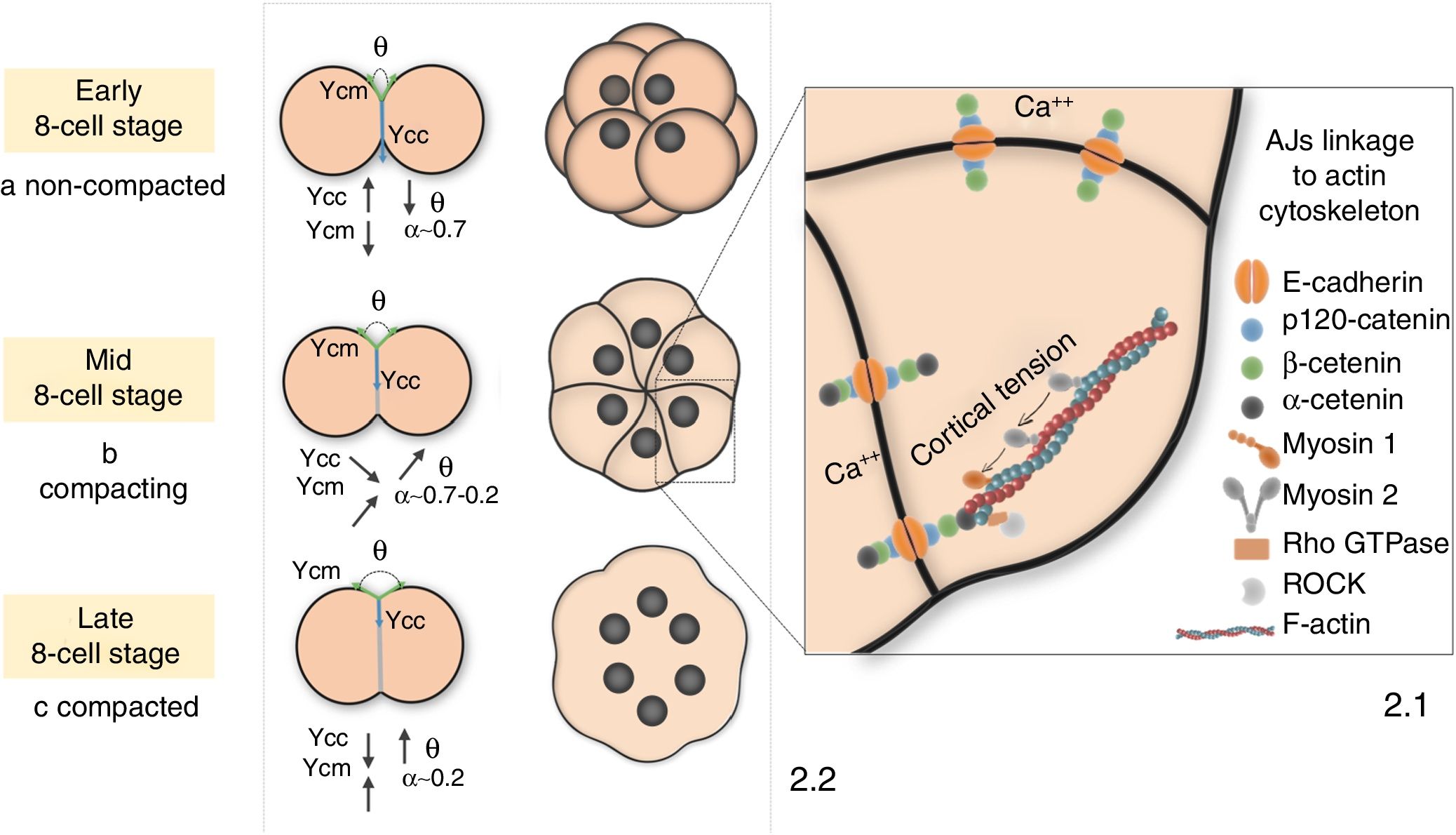
AJs link to the cell actin cytoskeleton by catenins and other proteins such as myosin 1 (Myo-1) (Diz-Munoz et al., 2010; Nambiar et al., 2009) or ezrin/radixin/moesin (ERM) (Kunda et al., 2008). This interaction is necessary to accomplish the drastic shape changes of compaction, allowing the transmission of forces generated by cytoskeleton-associated motor proteins (mostly nonmuscle myosin 2 (NM2)) toward the cell surface (Fig. 2.1). This cell-autonomously generated cortical tension is transmitted onto neighboring cells through the projection of adhesive filopodia (Fierro-Gonzalez et al., 2013). Besides, since E-cad plays a major role as biochemical transducer, cells can regulate the activity of the actin cytoskeleton (Lecuit and Yap, 2015; Maitre and Heisenberg, 2013) or other related proteins such as Rho, Rac and Cdc42 (Noritake et al., 2005; Clayton et al., 1999) according to extracellular signals, and thus regulate cortical tension and compaction mechanics.
Molecular and mechanical hallmarks of mouse embryo compaction. (2.1) AJs are bound to the cell actin cytoskeleton by p120, α and β catenins and other proteins such as myosin 1 or Rho GTPases. During compaction, NM2 pulls on this network to generate cortical tension, which is transmitted to the cell surface to deform the membrane. (2.2) (a) Before compaction, loosely bound membranes show low cell-surface tension (γcm) and high cell-interfacial tension (γcc), and hence α is closer to 1. (b) As compaction progresses, interface spreading minimises the γcc/γcm ratio and α falls between 0.7 and 0.2. (c) α drop close to 0 upon compaction completion due to a twofold γcm increase and a 1/3rd γcc decrease.
Taken together, these findings highlight the key role of AJs in embryo compaction. However, the exact function of the AJs components remains unknown. Given that they are all found at earlier stages (Levy et al., 1986), and that E-cad and β-catenin phosphorylation has been observed at the time of compaction (Clayton et al., 1993), it is plausible that compaction initiation is regulated through post-translational modifications (Aghion et al., 1994). However, the initial activating signal of the process remains obscure.
Embryo compaction results from the concurrent application of distinct forcesThe cortical tension generated by the actin cytoskeleton is believed to be the main force driving the cellular shape changes of compaction (Dai et al., 1999). Other minor sources of surface tension are the plasma membrane (Diz-Munoz et al., 2010; Hochmuth and Marcus, 2002; Nambiar et al., 2009; Dai and Sheetz, 1999) and cell adhesion (Maitre et al., 2012; Stirbat et al., 2013). Therefore, from a biomechanical perspective, compaction is the process by which the simultaneous application of adhesive and contractile forces (Maitre et al., 2012; Stirbat et al., 2013) deform membranes. These forces promote, respectively, the propagation and shrinkage of the cell contact interfaces. Despite being of a different nature, adhesion and contraction play exactly opposite roles, and hence both can be defined in the same units (force per unit length) and added up as tensile forces with opposite sign. Thus, unification of different mechanical contributions allows the quantification of the total stimulus required for membrane deformation and compaction (Turlier and Maitre, 2015).
In this light, the degree of compaction is defined by the Young-Dupré equation as α=γcc/2γcm=cos(θ/2). γcm, i.e. cell-medium surface tension, refers to the sum of forces exerted at the contact-free surfaces of the embryo; γcc, i.e. cell–cell interfacial tension, comprises forces exerted at the intercellular contact regions; and θ is the interblastomeric angle. α is a dimensionless parameter that ranges from 0 to 1 according to the degree of compaction (Turlier and Maitre, 2015) (Fig. 2.2). The quantitative estimation of α parameter represents a valuable approach to objectively monitor the course of compaction, which may help to unveil its underlying molecular regulation and its functional integration with other concomitant processes like polarization.
Cell polarization is established sequentially at the 8-cell stageCell polarization in the mouse embryo is initiated at the early-mid phase of the 8-cell stage, and entails the unequal distribution of certain cellular components along the apical-basal axis of the cell (Fleming and Johnson, 1988; Johnson and Maro, 1985).
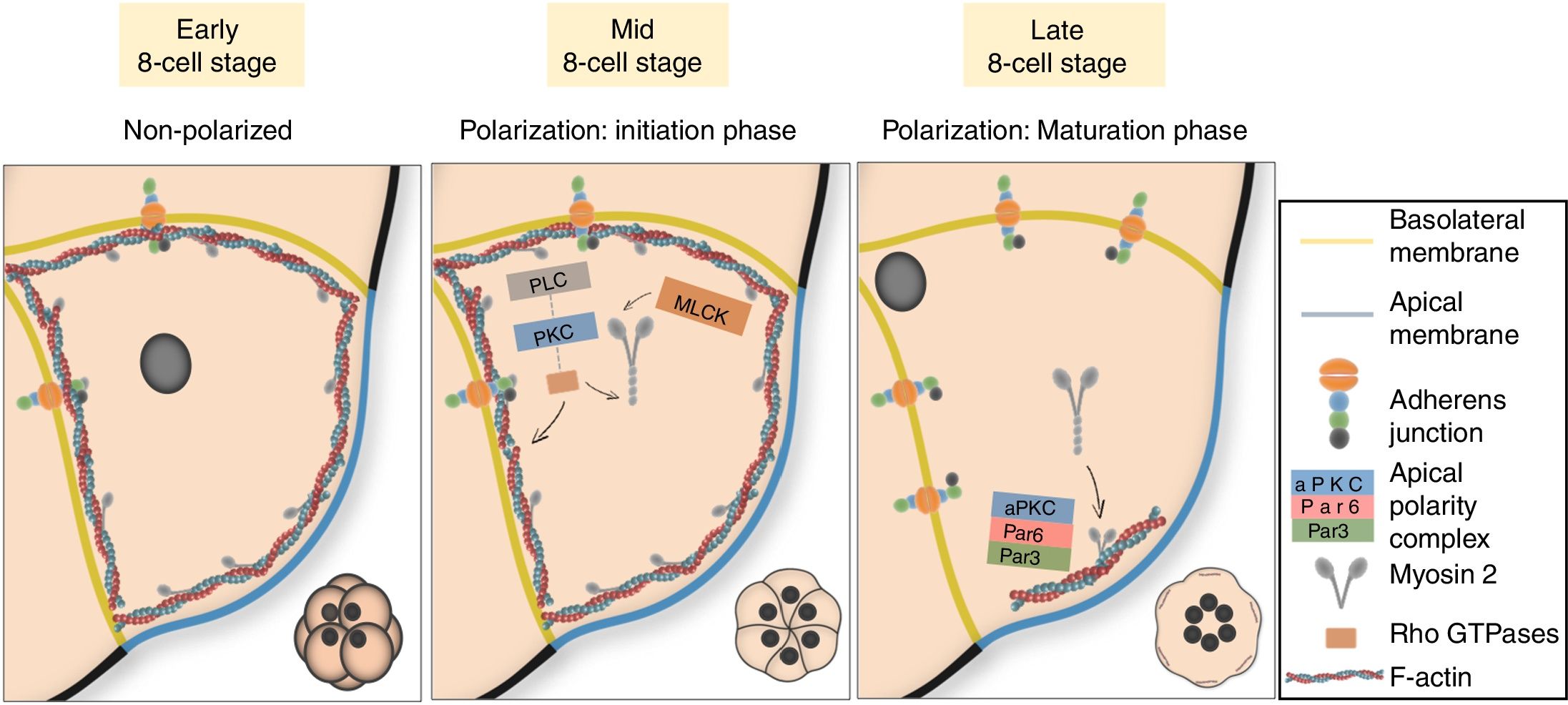
Two sequential stages define cell polarization in the mouse. The initiation phase runs in parallel with the start of compaction, and implies the cortical enrichment of actin and myosin underneath the contact-free surface of the embryo (Sobel, 1983; Zhu et al., 2017). Polarization of the cytoskeleton is followed by additional apical recruitment of microvilli and a number of cellular components, namely members of the ERM protein family, Par proteins (Par3-Par6) and different isoforms of the atypical protein kinase C (aPKC). Par-3-Par6-aPKC proteins build the apical polarity complex, which exclude actomyosin and establish a mature functional domain in the center of the cell apical region (Ajduk and Zernicka-Goetz, 2016; Vinot et al., 2005). On the non-exposed sides of the cell, polarization implies basal localization of the nucleus (Ajduk et al., 2014) and basolateral enrichment in adhesion and polarity proteins, mainly components of the AJs and Mark2 (mammalian homologue of Par1). Polarization of other unassembled junctional complexes such as gap or tight junctions can also occur at this stage, although these are not believed to participate in embryo compaction at the 8-cell stage (Fleming et al., 2000). Once compaction has been completed, the actomyosin network forms a ring-like structure around the mature apical domain (AD) (Zenker et al., 2018; Zhu et al., 2017). The cortical network of actomyosin and the polarization proteins constitute a conserved system in embryos from many species to establish cell polarity (Nance, 2014; Bornens, 2008; Munro, 2006; Campanale et al., 2017; Mullins, 2010; Sauvanet et al., 2015).
Actomyosin polarization is regulated by the PLC–PKC signaling pathwayA logical question at this point would be how do cells dictate the partitioning of their subcellular components during polarization. Is it E-cad-mediated cell communication or just contact what cells need to establish their domains? Interestingly, correct positioning of apical and basolateral domains during cell polarization does not require E-cad but is thought to rely on the mechanical stimuli of cell-to-cell interactions (Korotkevich et al., 2017). Orientation is achieved by activation and preferential allocation of myosin on the opposite side of the cell contact point, where it joins the cortical network of actin filaments (Zhu et al., 2017).
Apical actomyosin accumulation is known to be regulated by the PLC–PKC signaling pathway (Winkel et al., 1990; Ohsugi et al., 1993; Zhu et al., 2017). The PLC-mediated PIP2 hydrolysis results in the activation of PKC through the production of DAG. Actin polarization through PKC requires GTPases RhoA and Cdc42 (Clayton et al., 1999), while myosin activation and polarization demands not only GTPases activity but also the phosphorylation of its regulatory light chain (MRLC) by a specific kinase (MLCK) (Pichaud et al., 2019; Zhu et al., 2017) (Fig. 3). Artificial activation of PKC and/or exogenous expression of RhoA or Cdc42 in blastomeres from 4-cell stage embryos leads to premature actomyosin polarization and compaction, but not to AD formation (Clayton et al., 1999; Zhu et al., 2017). This means that the establishment of a mature apical cap needs additional factors still not present at the 4-cell stage. The GTPase-dependent Rho-associated kinase (ROCK) emerges as a potential candidate, as this protein is known to regulate ERM (Amano et al., 2010) and its inhibition in 8-cell stage embryos prevents AD formation (Kono et al., 2014). RhoA inhibition with C3-transferase before the 8-cell stage prevents actomyosin polarization, intercellular flattening and compaction; while treating embryos with cytochalasin D, an actin inhibitor, impairs compaction but not polarization (Clayton et al., 1999). Thus, it is also evident that although polarization runs in parallel to compaction at the 8-cell stage, both processes are subject to different regulatory mechanisms. Although the molecular map of cell polarization is now starting to be deciphered, what triggers the contact-induced activation of the PLC–PKC signaling route at a timely moment of embryo development remains a complete mystery.
Cell polarization in the 8-cell mouse embryo. Polarization is initiated with the PLC–PKC-mediated enrichment of the actomyosin cytoskeleton at the apical cell cortex. This is followed by additional apical recruitment of aPKC and Par proteins during the maturation phase, which build the apical polarity complex. Conversely, cell nuclei allocate basally, and basolateral surfaces become enriched in adhesion proteins.
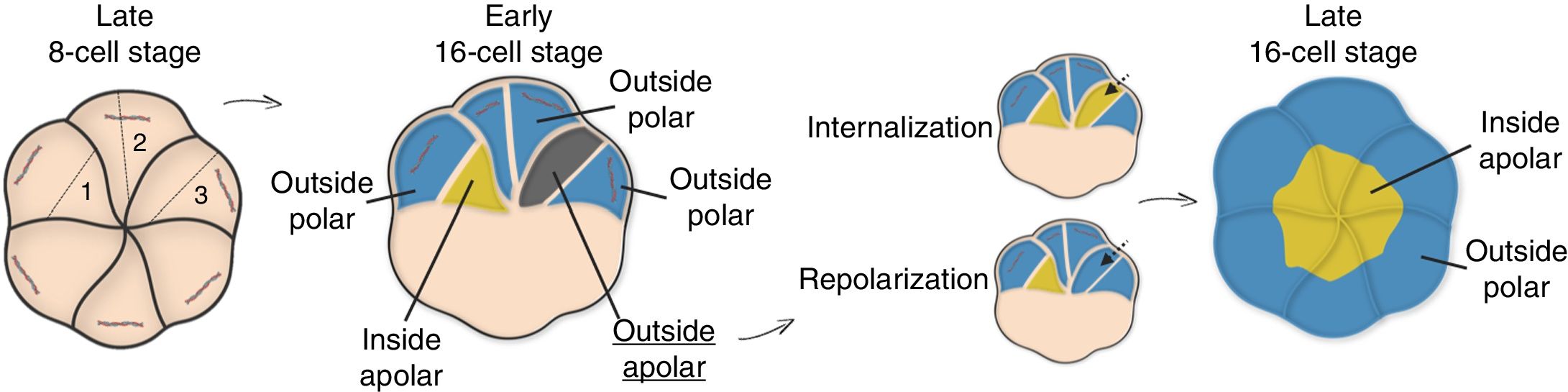
As seen, the irregular partitioning of certain subcellular components at polarization leads to the formation of functional domains that break intracellular symmetry in the late 8-cell embryo (Thomas et al., 2004; Plusa et al., 2005; Vinot et al., 2005). Following polarization, asymmetric divisions of the polarized blastomeres lead to intercellular symmetry breaking, and hence, cells arising from the 8 to 16 cell transition can be categorized according to two properties: position and molecular inheritance. Inside cells are those embedded in the core of the embryo, while outside cells present an exposed surface. Additionally, cells that inherit any of the AD remain polar, whereas daughter cells lacking an AD are apolar. Inside cells can only be apolar, whereas outside cells can be either.
The early 16-cell embryo has a mean relative composition of 1–2 apolar internal cells, 10 polar external cells and 4–5 apolar external cells, although these numbers can vary (Posfai et al., 2019). New questions arise: How do polarized cells control the orientation of their division planes? Is there any control over these axes or are cells cleaved randomly? AD has been proposed to regulate the orientation of the mitotic spindles at the 8-cell stage, since it directs the subcortical recruitment of the microtubule organizing centers (MTOCs) (Korotkevich et al., 2017). In addition, cells lacking an AD exhibit random divisions (Korotkevich et al., 2017), and those with a larger AD show a greater tendency to generate only polarized cells through symmetric divisions (Pickering et al., 1988). However, the AD seems to disassemble prior to division and reassemble again at the 16-cell stage (Zenker et al., 2018), so it is not clear how AD would influence division. Other studies suggest that the tendency of a blastomere to divide asymmetrically is influenced by the duration of intercellular contact events (Humiecka et al., 2017), the cell membrane (Dard et al., 2009), the cytoskeleton (Fleming, 1987) or the nucleus (Ajduk et al., 2014), but so far none of these mechanisms is conclusive.
Just before starting a new round of division, the 16-cell embryo has an average composition of 4–5 apolar internal cells and 10–11 polar external cells (Posfai et al., 2019). This new embryo layout is achieved through division-independent reorganization events that sort out cells into two populations during the 16-cell stage (Fleming, 1987; Suwinska et al., 2008) (Fig. 4). In most cases, dynamic sorting is explained by internalization of apolar cells (Samarage et al., 2015; Korotkevich et al., 2017; Maitre et al., 2016; Anani et al., 2014), although occasional repolarization events can also occur (Anani et al., 2014).
Dynamic sorting at the 16-cell stage. Depending on the orientation of the cleavage axis at the 8 to 16 cell transition, the freshly formed cells can be inner (if embedded in the embryo core), outer (if they have an exposed surface), polar (if they inherit any of the AD) or apolar (if they inherit none of the AD). Division plane 1 generates polar outside and apolar inside cells. Division plane 2 generates only polar outside cells. Division plane 3 gives rise to outside polar an outside apolar cells. Dynamic sorting ensures that position matches with polarity: outside apolar cells internalize or repolarize to transform into inside apolar or outside polar cells, respectively. The late 16-cell stage embryo has two cell populations: inside and outside polar cells.
Dynamic sorting obeys different rules. Apolar cells show greater contractility than polar cells (Anani et al., 2014). Interestingly, inhibition of the cytoskeleton contractility (Samarage et al., 2015; Maitre et al., 2016; Anani et al., 2014) or AD transplantation (Korotkevich et al., 2017) blocks the internalization of apolar outer cells, which suggests that the AD may influence sorting by regulating actomyosin contractility. In contrast, isolation of apolar inner cells leads to their repolarization (Johnson and Ziomek, 1983; Eckert et al., 2004). This multifaceted regulation of dynamic sorting is consistent with classical observations (Tarkowski and Wroblewska, 1967; Johnson and Ziomek, 1981a,b). Internalization means cells are sorted according to their polarity state, while in repolarization cell position is the prevailing criterion. However, the reason why some cells internalize and other repolarize is not known (Fujimori et al., 2009; Anani et al., 2014; Dietrich and Hiiragi, 2007). The 16–32 cell transition can still generate cells that need further rearrangement, although there is disagreement about the frequency of these events and the ultimate fate of the arising cells (Strnad et al., 2016; Watanabe et al., 2014; Morris et al., 2010; Yamanaka et al., 2010).
Lineage differentiation is initiated at the 16-cell stageDynamic sorting ensures that cell polarity matches with position at the late 16-cell stage, but what for? Throughout the 16-cell stage, pluripotency becomes gradually restricted to a few inner cells confined within the embryo core, which means that for the first time during embryo development, cell fate bifurcates to establish separate lineages. Thus, inner cells will build the ICM, which will make the embryo proper and the yolk sac; while outer cells will develop into the TE, which will lead to most of the extraembryonic tissues of the placenta (Posfai et al., 2017, 2019; Korotkevich et al., 2017; Hirate et al., 2013).
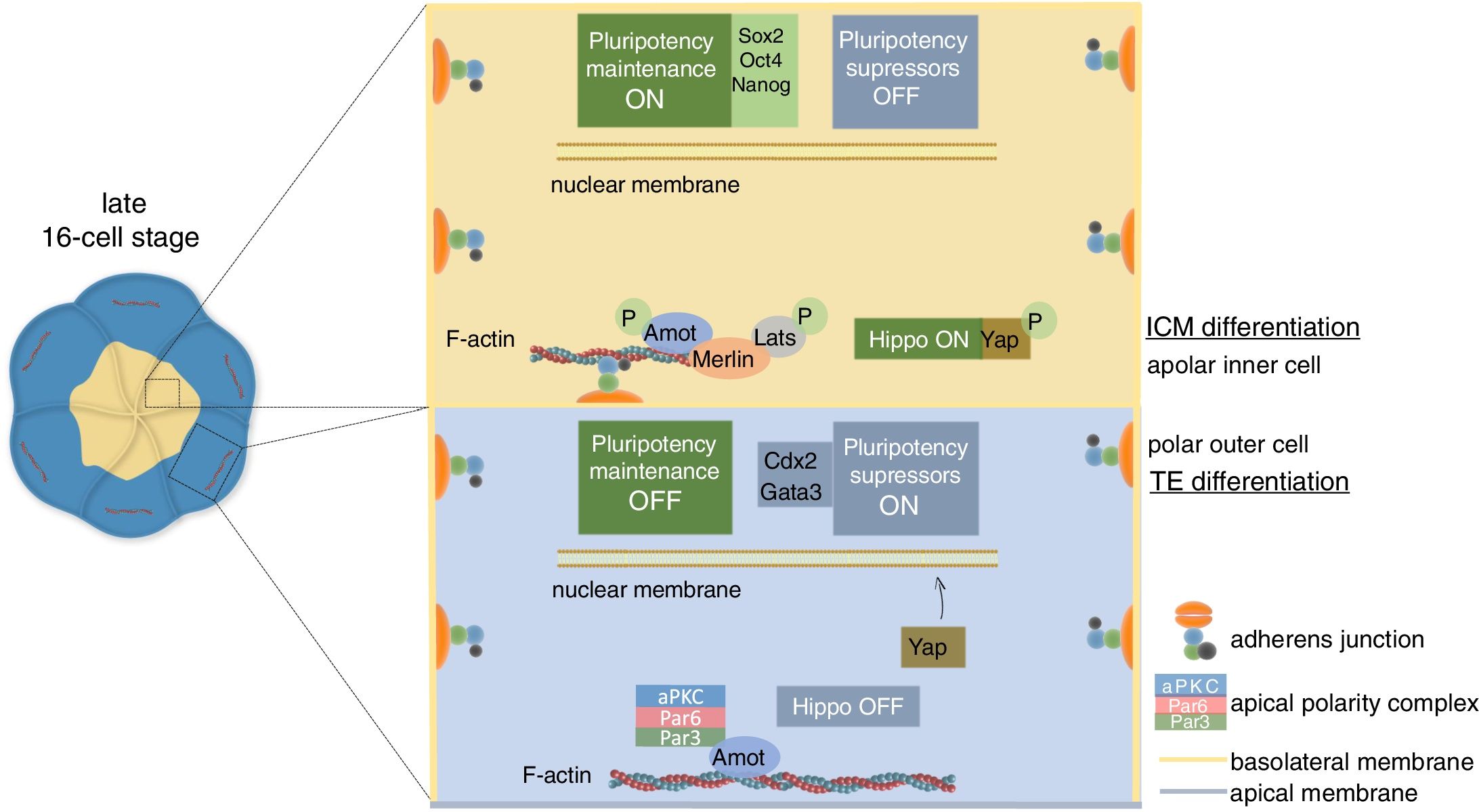
This very first cell fate decision to contribute to either the ICM or the TE is achieved by integration of two types of information: position, which is inferred by the cell contact pattern; and polarity. This is only possible thanks to a key cytoskeleton-associated protein called angiomotin (Amot), which converts the mechanical stimuli of cell contact into signals that activate or repress differentiation pathways depending on cell polarity.
In apolar inner cells, Amot is linked to junctional E-cad via the cytoskeleton (Hirate et al., 2013). The adhesion stimulus induces phosphorylation and activation of Amot, which recruits members of the Hippo signaling pathway to the adhesion site resulting in the activation of the route. Activation of the Hippo pathway favors the cytoplasmic retention of Yap, a nuclear transcriptional activator of the expression of pluripotency suppressor genes. Since Yap cannot enter the nucleus, these cells retain pluripotency and become ICM by expression of pluripotency transcription factors, mainly Sox2, Oct3/4 and Nanog. In polar outer cells, the adhesion-mediated activation of the Hippo pathway is prevented by the apical polarity complex (i.e., Par3-Par6-aPKC), which induces apical Amot sequestration away from the cell–cell contacts. This means that although adhesion events actually occur at the basolateral interfaces, Amot cannot detect them nor activate the Hippo pathway. Thus, Yap is released from the cytoplasm to enter the nucleus, where it interacts with Tead4 to activate the expression of pluripotency suppressor genes, particularly Cdx2 and Gata3. As a consequence, these cells will initiate their differentiation into the TE (Campanale et al., 2017; Leung and Zernicka-Goetz, 2013; Alarcon, 2010; Sasaki, 2017; Hirate et al., 2013) (Fig. 5).
ICM/TE cell lineage specification in the mouse embryo. In inner cells lacking polarity, Amot is linked to basolateral AJs. Cell contact induces Amot phosphorylation and activation, which recruits Merlin, Lats and other components of the Hippo pathway to the site of adhesion. Activated Lats phosphorylate Yap, which is retained in the cytoplasm. Consequently, the cell expresses pluripotency maintenance factors, mainly Sox2, Oct4 and Nanog, and the cell specifies to ICM. In polarized outer cells, Amot is sequestered by the apical polarity complex, and hence Hippo signaling cannot be activated. Unphosphorylated Yap can enter the nucleus to activate the expression of pluripotency suppressor genes, mainly Cdx2 and Gata3, which direct cell specification to TE.
The formation of specialized cell types during embryonic development permits the generation of a complete organism from a single totipotent cell. The gradual restriction of cell potential culminates with differentiation, a process through which cells turn on the expression of a specific gene set in order to acquire the morphology and functions, and thus the identity, of particular cell types. However, differentiation is preceded by gradual cell commitment, by which cells engage to a specific differentiation program without altering their phenotypic profile. Thus, cells can be specified (if autonomous and reversible differentiation occurs in a neutral environment) or determined (if autonomous and irreversible differentiation occurs in an environment favoring any other fate) (Cooke, 1992; Harrison, 1933).
At least until polarization, early blastomeres can contribute to any lineage. However, can embryos be considered a wholly homogeneous mass of identical cells until the 8-cell stage? When do cells lose plasticity and become unable to morph into one of the two lineages? These have been key questions in the field for decades. In the mouse embryo, commitment of TE and ICM progenitor cells is asynchronous. In TE precursors, specification and determination occur simultaneously, starting primarily at the 16-cell and mostly at the early 32-cell stage. Although ICM precursors are similarly determined at the 32-cell stage, commitment is not acquired until the 64-cell stage. Plasticity retention of the ICM and TE commitment may ensure that a sufficient TE cell number is achieved before implantation (Posfai et al., 2019), although the molecular mechanisms behind the temporal separation of these events remain elusive. Other studies have reported a link between early heterogeneities and the tendency to become a particular cell type (Chazaud and Yamanaka, 2016), which opens the door to the identification of new polarity cues that may influence cell fate from early stages of development.
DiscussionThe last decade has seen a steady improvement in the effectiveness of human assisted reproduction techniques, which have led to a considerable reduction on the time to achieve a pregnancy and a live birth (Wade et al., 2015). Growing success rates have been driven by continuous advances in embryo culture conditions (Swain et al., 2016), and by the development of novel technologies that allow selection of euploid embryos for transfer, which display higher implantation potential compared to their aneuploid counterparts (Munne et al., 1995). However, since we still miss key information about both uterine and embryonic factors contributing to the achievement and maintenance of a normal pregnancy (Hur et al., 2019; Reljic et al., 2017), success rates still have room for improvement.
The general outline of early embryogenesis is common to most mammals, with some conserved morphogenetic events leading to the formation of a morphologically similar blastocyst, this consisting of different tissue-like structures committed to build up a certain lineage.
Recent approaches made to gain deeper insights into human preimplantation development have unveiled similarities but also fundamental differences with regard to other mammalian species. For instance, time-lapse imaging (TLI), which allows the continuous monitoring of preimplantation embryo development from the zygote to the blastocyst stage, have revealed that events leading to blastocyst formation generally appear at a slightly higher cell number in the human – for example, compaction and cavitation occur roughly at the 8–16 cell and at the 64-cell stage, while in mouse embryos these happen at the 8-cell and at the 32-cell stage, respectively (Cockburn and Rossant, 2010).
Other differences may reside in the molecular mechanisms governing compaction, polarity and cell lineage specification, despite they seem to be evolutionarily conserved pathways among phyla (Ajduk and Zernicka-Goetz, 2016).
Studies on TE and ICM specification, partly related to inside and outside cell positioning and orientation, show differences among species. In the mouse embryo the development of TE and ICM are separated in time, taking place at the 16 cell and at the 32-cell stage, respectively (Posfai et al., 2017). In humans, single-cell transcriptome analysis has evidenced that lineage specification of TE and ICM precursors seem to occur almost simultaneously but at a later stage, coinciding with blastocyst formation (Petropoulos et al., 2016). However, recent experiments combining network analyses, dimensional scaling and change point analysis have shown that despite the majority of 8-cell human blastomeres are still pluripotent and unlikely to be lineage-specified, they are not transcriptionally equivalent as some may display diverging tendencies toward formation of the TE or ICM (Smith et al., 2019). In the mouse the lack of transcriptional equivalence occurs as early as the 2–4 cell stage (Biase et al., 2014).
Interspecies discrepancies with human embryos may arise not only from a temporal viewpoint, but also regarding the transcriptional networks involved in the outbreak, establishment and maintenance of the different cell lineages. Transcriptome analysis of blastocysts has also revealed different expression signatures in TE and ICM cells of human embryos compared to the mouse. Human TE cell lineage is transcriptionally distinguished by a marked expression of the genes KRT18/8, EFNA1, DAB2, PPARG, GATA2/3, TEAD3 and FHL2 (Stirparo et al., 2018), while other genes such as EOMES and ELF5 show no transcriptional activity (Blakeley et al., 2015). Instead, TCFAP2C is expressed in both human TE and ICM cells at similar levels, while in the mouse embryo expression of this gene is enriched in TE cells (Blakeley et al., 2015).
Compaction and polarization represent two crucial developmental milestones of mammalian embryogenesis, during which the embryo must make major decisions with respect to cell position and fate that will impact its developmental potential. Accordingly, a close relationship between a timely embryo compaction, blastocyst morphology and successful implantation has been found in human embryos (Skiadas et al., 2006; Mizobe et al., 2017; Motato et al., 2016). However, overall, our degree of understanding about the molecular basis and the transcriptional features of the morphogenetic events that lead to the first lineage bifurcation in the human embryo is still at a very incipient stage. While these are believed to be driven by the interplay among the cytoskeleton, Par signaling and intercellular interactions as seen in other species, further studies are urgently needed to explore the underlying molecular regulation. This information may uncover still obscured sources of failure, potentially aiding IVF practitioners in the improvement of embryo culture conditions or embryo selection, and thus opening a new window of opportunities to patients suffering from embryonic-derived infertility issues.
ConclusionsCompaction and polarization are concomitant morphogenetic events key to establish the first two lineages of the developing embryo. De novo polarized blastomeres at the 8-cell stage give rise to cell populations with distinct polarity after a round of asymmetric divisions, which are then reorganized to establish the outer/inner configuration of the embryo. The position-dependent activation of the Hippo pathway relies on the simultaneous integration of two types of information: contact and polarity; and triggers the expression of a gene set that build a particular cellular identity, i.e., ICM or TE. Although the sequence of events leading to lineage specification is becoming better known, some core questions keep unresolved: How are compaction and polarization triggered? Are early blastomeres equivalent in potency, fate and gene expression? How do cells lose plasticity? Do polarized blastomeres control the frequency of their asymmetric divisions? What criterion uses the cell to internalize or repolarize during dynamic sorting? Bearing in mind that fundamental species-specific differences may exist, answering these questions will help to improve the IVF outcome of patients suffering from idiopathic infertility.
FundingThis manuscript has not been funded by any organization.
Conflict of interestThe author declares that there is no conflict of interest regarding the publication of this manuscript.
The authors would like to express their gratitude to Dr. Pilar Buendía for her implication and Ms. Aina Baixauli for her help in creating the figures of this manuscript.