In this study, we analyzed the conservation of a semi-liquid bio-preserver (SL778) developed with Lactobacillus plantarum CRL 778, a lactic acid bacterium (LAB) having antifungal activity. The characteristics of the SL778 starter remained stable during a 14-day storage at 4°C. At −20°C, cell viability and organic acid concentration showed a significant (p<0.05) decrease after 7 days. These differences observed between the storage temperatures tested were reflected in the acidification activity of SL778 during dough fermentation. However, SL778 maintained its antifungal efficacy up to a 14-day storage at both temperatures. Sensory attributes (acidic and spicy tastes and acidic smell) of breads manufactured with starter SL778 (stored at 4 or −20°C) were evaluated. No undesirable difference was detected with respect to bread control without SL778 and bread manufactured with SL778 (stored at 4 or −20°C). In conclusion, the SL778 semi-liquid bio-preserver can be stored at 4 or −20°C without modifying its antifungal activity during 14 days.
Se evaluó la estabilidad de un bioconservante semilíquido destinado a panificados envasados, desarrollado con la bacteria láctica con actividad antifúngica Lactobacillus plantarum CRL 778. Las características del bioconservante, designado como SL778, se mantuvieron estables durante 14 días de almacenamiento a 4°C. A –20°C, la viabilidad celular y la concentración de ácidos orgánicos disminuyeron significativamente (p<0,05) después de 7 días de almacenamiento. Estas diferencias según la temperatura de almacenamiento se reflejaron en la actividad acidificante de SL778 durante la fermentación de las masas. Sin embargo, SL778 mantuvo su eficacia antifúngica por hasta 14 días con el almacenamiento a ambas temperaturas. Se evaluaron los atributos sensoriales de los panificados elaborados con SL778 (gusto ácido y picante y olor ácido) tras el almacenamiento a las dos temperaturas. En tal sentido, los panelistas no detectaron diferencias que vuelvan al producto indeseable al comparar los panificados control (sin SL778) y los elaborados con SL778, tanto almacenados a 4°C como a –20°C. En conclusión, el bioconservante semilíquido SL778 se puede almacenar a 4°C o a –20°C durante 14 días sin que ocurran cambios en su actividad antifúngica.
Fungal spoilage is the main cause of substantial economic losses in the baking industry21. Fungi growth in baked goods is influenced by several factors, for example, the type of product (bread or sweet baked goods), the ingredients, the starter culture, the bakery layout and the packaging of the products, among others. Since fungal spores are killed during baking (cooking step), fungal spoilage is due to airborne molds which contaminate the baked goods during cooling, slicing, wrapping and storage operations. Salts of propionic, sorbic and benzoic acids are frequently used as chemical preservatives. Most bakeries in Argentina use calcium propionate (CPa) at the maximum concentration (0.4g CPa/100g wheat flour) allowed for packed sliced breads by the Argentine Food Code (AFC); however, results are not always satisfactory. Moreover, the reduction in NaCl decreased the bread shelf-life by 2 days1,13.
The bio-preservation or the use of microorganisms and/or their metabolites to prevent spoilage and to extend food shelf life has gained the interest of producers due to the consumers’ demands24. Among the protective microorganisms, lactic acid bacteria (LAB) are of particular interest because it is well documented that they are capable of producing many molecules with fungicidal effects5,19,20,24. Some studies have demonstrated the LAB antifungal activity under laboratory conditions10,14–16, whereas other investigations were aimed at highlighting the capacity of antifungal LAB strains to actually prevent mold growth in leavened baked goods4,8,9,18,19,24. The research on bio-conservation for packed bread has been ongoing in our laboratory since 2006 and a ready-to-use semi-liquid bio-preserver (SL778) was developed by using the antifungal strain Lactobacillus plantarum CRL 77811. The antifungal activity of this strain was related to the presence of lactic, acetic and phenyllactic (PLA) acids and was dependent on pH value. In addition a synergistic effect was observed when SL778 and CPa were added to the bread formulation9.
Methods used in the preservation of lactic starter cultures have involved low-temperature storage, freezing, and freeze-drying under various conditions and with various protective additives. This study evaluates the stability of the SL778 starter along their storage at 4 and −20°C, focusing on chemicals, microbiological and preservative aspects linked to the antifungal activity. Sensory attributes of bread manufactured with stored starter were also tested.
Materials and methodsProduction and conservation of the SL778 bio-preserverThe selected strain L. plantarum CRL 778 used in this study belongs to the Culture Collection (CRL) of the Centro de Referencia para Lactobacilos (CERELA), Tucumán–Argentina. Before experimental use, the strain was grown at 37°C for 16h in MRS broth6 without sodium acetate to avoid overlapping of the antifungal metabolites produced by the starter. The culture was inoculated (20ml/100ml) in a mixture of (g/l) 325 wheat flour 000 type, 5 sucrose, and 12 skimmed milk powder; tap water, 1l.
The mixture was homogenized manually and the pH was adjusted to 5.90±0.13 with Na2HPO4. Fermentations proceeded at 37°C for 16h under stirring and free-pH conditions.
The semi-liquid bio-preserver SL778 obtained was fractioned and stored at 4 and 20°C. Samples from 0, 7, 10, and 14 days of storage were withdrawn for microbiological, chemical and antifungal studies. LAB viability was determined by the plate dilution method pouring proper dilutions with MRS agar into Petri dishes; the plates were incubated at 37°C for 48h, and results were expressed as log CFU/ml. pH measurements (Altronix-TPX1 pH/mV-Meter, Sartorius, Goettingen, Germany) and total titratable acidity (TTA), measured by using the potentiometric method with Dornic solution, were evaluated as parameters of acidity. Organic acids linked to the antifungal activity in vitro9 were determined by High Performance Liquid Chromatography (HPLC) as described below.
Organic acid determinationsSamples from stored SL778 were centrifuged (12,000g, 10min) to obtain a clarified supernatant for HPLC analysis (Knauer Company, Berlin, Germany). The protein content was removed by precipitation (4°C for 30min) with trichloroacetic acid (final concentration 16g/100ml), followed by centrifugation (12,000g, 15min at 5°C), filtration (0.22μm filters; Ministart high flow, Sartorius, Goettingen, Germany) and injection (20μl) into an Aminex HPX-87 H column (300mm×7.8mm, Bio Rad, USA). An isocratic mobile phase (H2SO4 5mmol/l) at flow rate 0.6ml/min and a column temperature of 45°C were used to elute the samples. A refractive index detector was used to identify lactate and acetate, while an UV detector set at 210nm was used to identify phenyllactic acid. Both detectors were connected to the Peak Simple II software for data analysis. Organic acid concentrations were expressed in mmol/l.
Dough fermentation and bread manufactureThe bio-preserver SL778 stored at 4 and −20°C was used for the manufacture of wheat bread. Doughs were prepared as follows: 1000g commercial wheat flour 000 type, 10g NaCl, 20g sucrose, 50g skim milk powder, 20g fat, 4g CPa and 0.6 l tap water. To incorporate the starter SL778, the tap water in the dough preparation was partially replaced (40ml/100ml water) by equal amounts of SL778. Dough prepared only with CPa (0.4g CPa/100g wheat flour) was used as control of contamination. Commercial yeast Saccharomyces cerevisiae (Calsa, Yeast Company Argentina S.A.), was used as leavening agent for all doughs (7 log CFU/g dough).
Ten dough pieces of 100g each were individually placed in aluminum pans for fermentation (2h, 30°C), pH variation (named as acidification activity) being determined in portions of 10g dough (Altronix-TPX1 pH/mV-Meter with electrode Hanna FC 210B for semi-solid samples). After fermentation, the doughs were baked in a batch oven (160°C for 45min).
Antifungal activity testsThe antifungal activity of SL778 stored at different temperatures was determined using bread slices obtained with a sterile knife. Each loaf was transversely sliced under sterile conditions to obtain 16 uniform slices of 10mm thickness and exposed to environmental pollution for 1h; then, packed into sealed polyethylene bags, stored at room temperature (25–30°C) and daily observed. The shelf life of bread slices was defined as the time (in days) for molds to become visible on the surface of the packaged loaves.
Sensory analysisSensory attributes of breads (acidic and spicy tastes, and acidic smell) were evaluated by an untrained panel (17 tasters) using a qualitative 5-point hedonic scale: 1 “very less”, 2 “less”, 3 “indifferent”, 4 “much” and 5 “very much” with respect to a control bread. The samples evaluated were: bread elaborated with 0.4% CPa and SL778 stored during 7, 10 and 14 days at different temperatures (4 and −20°C), and the control bread with only 0.4% CPa.
To evaluate the preference between the types of breads, the untrained panel also used a qualitative 5-point hedonic scale: 1 “very good”, 2 “good”, 3 “indifferent”, 4 “dislike slightly”, 5 “dislike very much”. Experimental samples were prepared 1h before serving and kept at room temperature (25°C). The breads were sliced into 10cm-long portions. The slices ends were not used. The panelists tasted approximately two slices of each sample wrapped in aluminum paper and identified with three-digit codes. Between samples, panelists rinsed their mouths with mineral water. Statistical analysis was carried out with respect to a control containing only CPa, by using the ANOVA test for mixed models (Infostat 2008p software).
Statistical analysisThree independent assays are presented as mean values±standard deviation (SD). Data were compared by ANOVA and the Dunnett t-test, and statistical significance (p<0.05) was determined (Minitab-12 software).
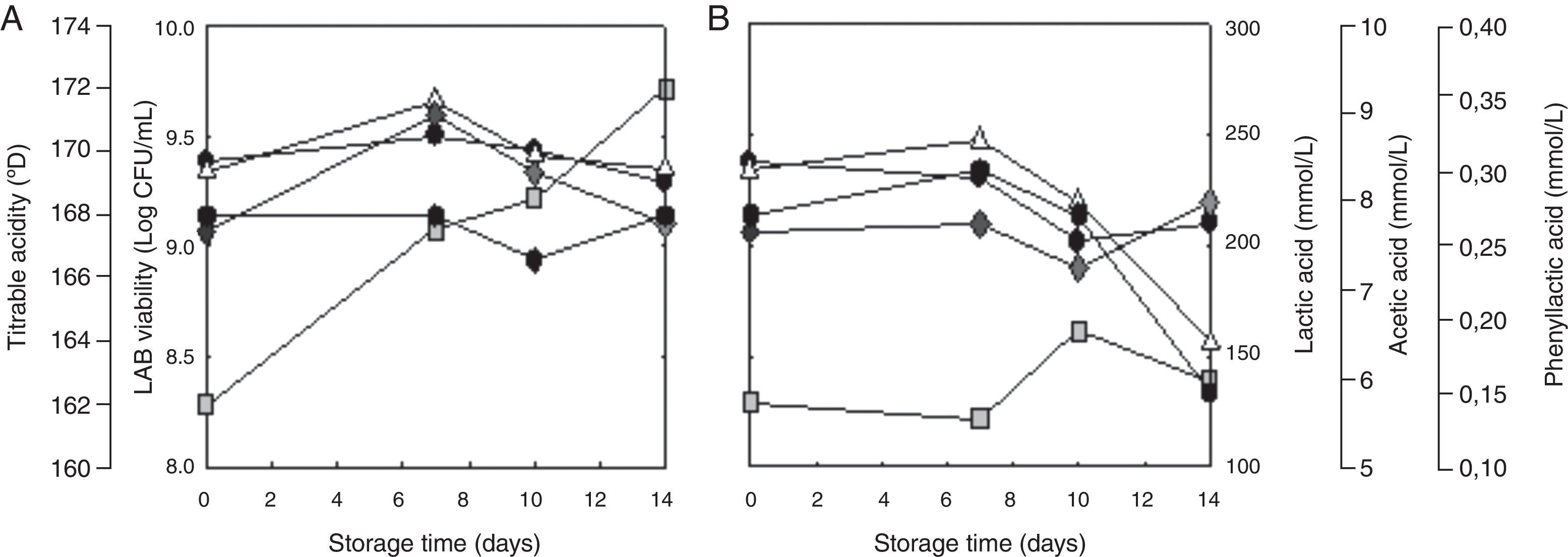
ResultsConservation of the SL778 semi-liquid bio-preserverAfter 16h fermentation, the starter SL778 was cooled rapidly to stop the acidification process, fractioned and stored under different conditions for 14 days. The characteristics of SL778 stored at 4 and −20°C are presented in Fig. 1. Until 14 days of conservation at 4°C, cell viability was constant while TTA showed an increment since the first day of storage. Organic acid concentrations varied slightly at 7 (acetic and lactic acids) and 10 (phenyllactic acid) days of storage (Fig. 1A). Nevertheless, these differences were not significant (p>0.05). The pH value (3.6) remained stable throughout the storage at 4°C (data not shown).
A different behavior was observed in SL778 stored at −20°C. Cell viability and lactic acid showed a significant (p<0.05) decrease after 7 day-conservation (Fig. 1B). On the contrary, no significant variations in TTA or in the pH were observed in this period.
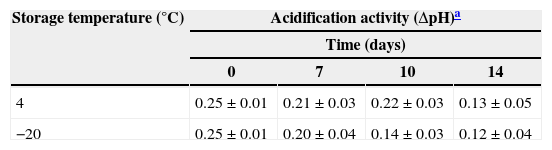
Acidification activity of the SL778 bio-preserver in the doughThe stored SL778 starter was added to the dough for bread manufacture and the variation of pH (ΔpH) value was determined during fermentation. Obtained results are shown in Table 1. The initial and final pH of the doughs containing fresh non-stored SL778 starter (day zero) were ca. 5.33 and 5.08, respectively, resulting in a ΔpH of 0.25. When the starter SL778 was stored at 4°C its activity in acidifying the dough (ΔpH 0.21–0.25) was maintained up to 10 days but decreased thereafter (ΔpH 0.13 at day 14). Unexpectedly, the activity of SL778 stored at −20°C was reduced after 7 days of storage.
Acidification activity of doughs fermented with the semi-liquid starter SL778 after its storage for 0, 7, 10 and 14 days under different conditions.
| Storage temperature (°C) | Acidification activity (ΔpH)a | |||
|---|---|---|---|---|
| Time (days) | ||||
| 0 | 7 | 10 | 14 | |
| 4 | 0.25±0.01 | 0.21±0.03 | 0.22±0.03 | 0.13±0.05 |
| −20 | 0.25±0.01 | 0.20±0.04 | 0.14±0.03 | 0.12±0.04 |
The initial pH of all doughs was ca. 5.30.
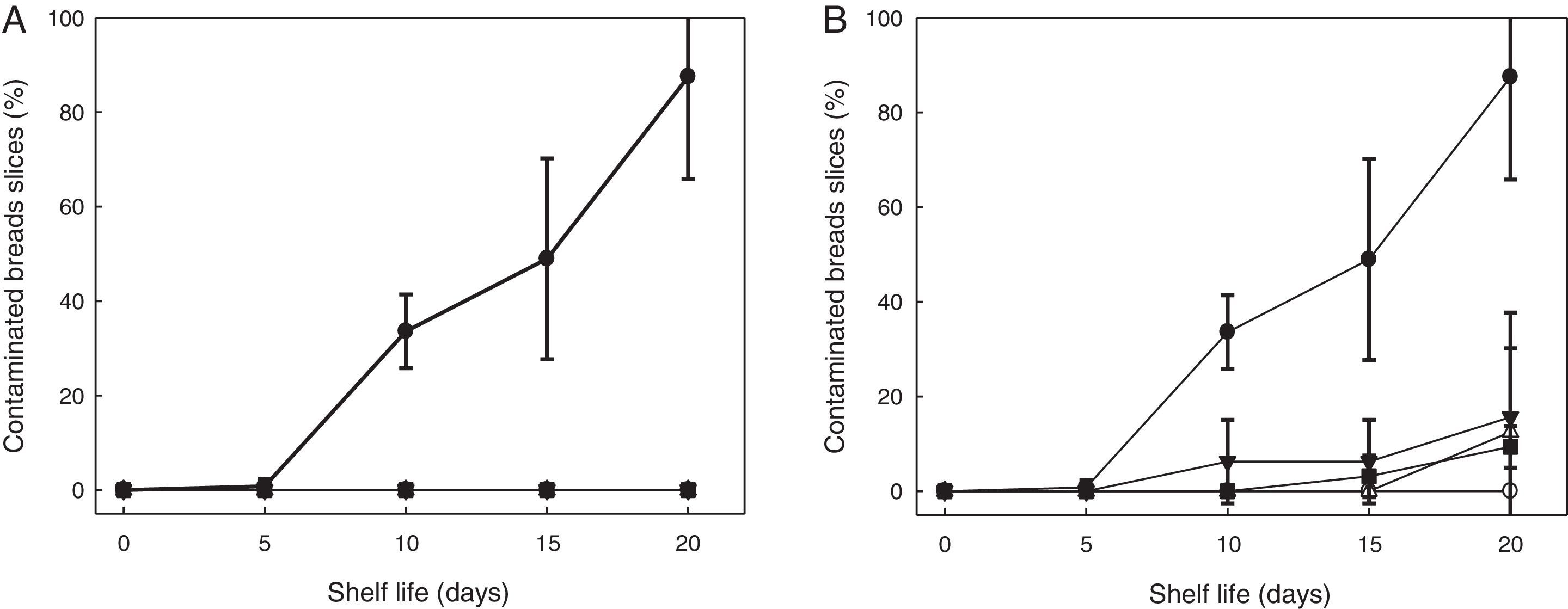
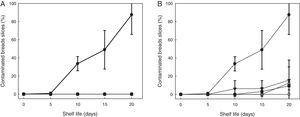
After storage for 7, 10 and 14 days at both temperatures, the SL778 starter was added at 24% to the dough and their ability to extend the shelf life was evaluated (Fig. 2). Comparison with the spoilage rate in the control breads (with only 0.4g CPa/100g wheat flour) revealed that the SL778 bio-preserver retarded spoilage of sliced bread. Slices of control breads were completely spoiled after 20 days, fungal growth was therein observed as from the sixth day. Compared to the control, the addition of the SL778 starter increased bread shelf-life to 20 days, regardless of the temperature and conservation time. No fungal growth was observed after 20 days in the bread containing SL778 stored at 4°C, while only 15% of the bread slices containing SL778 stored at −20°C showed fungal contamination in the same period.
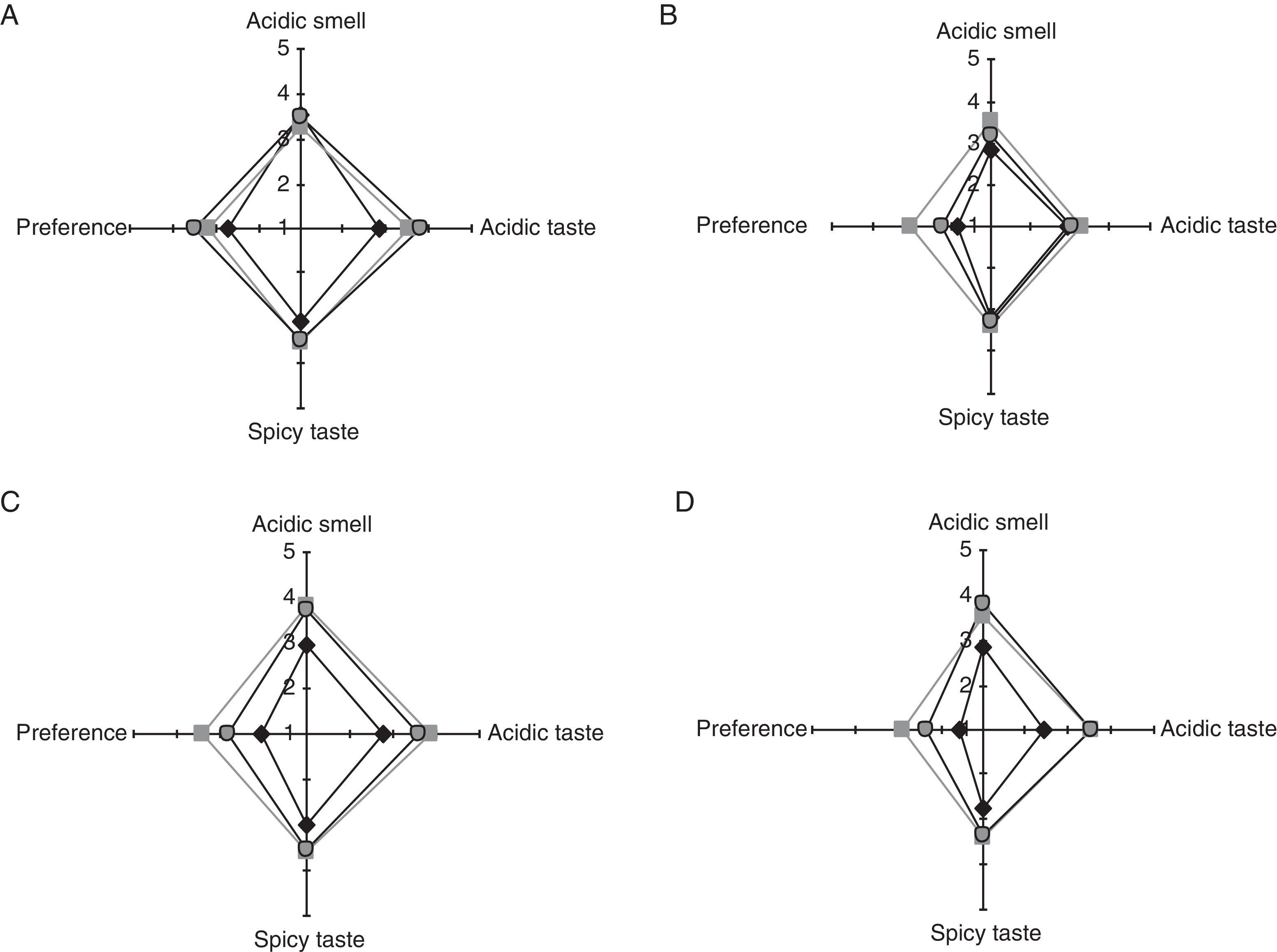
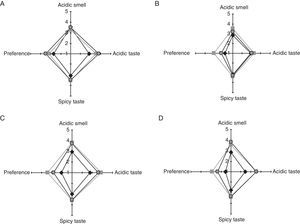
Sensory analysis of bread elaborated with stored SL778 semi-liquid bio-preserverSensory attributes of breads (acidic and spicy tastes, and acidic smell) fermented with SL778 stored at 4°C and −20°C during 7, 10 and 14 days were evaluated with respect to control containing 0.4g CPa/100g wheat flour. The untrained panel applied a qualitative 5 point-hedonic scale. Spider web diagrams of the mean scores of the sensory attributes of bread are depicted in Fig. 3. The study showed that the differences between breads elaborated with the SL778 starter and control breads could be perceived by an untrained panel. The acidic (3.3–3.9 points) and spicy (3.3–3.6 points) taste, and acidic smell (3.2–3.8 points) of breads containing SL778 starter, were considered quite higher than in the indifferent valuation (punctuation of 3.0), no significant difference (p>0.05) being observed regarding the temperature and time of SL778 storage. With respect to the preference, this feature was slightly lower (assessment between “good” and “indifferent”) for breads containing the SL778 starter than for the control bread containing 0.4g CPa/100g wheat flour (assessment between “very good” and “good”), regardless of the storage condition of SL778.
DiscussionBread is a staple food worldwide and it is generally considered a highly perishable product. Fungi spoilage markedly decreases bread shelf life causing huge economic losses to the baking industry and risks to consumer's health14. Chemical preservatives (propionic, sorbic and acetic acids and their salts) are often used to reduce mold growth in bakery goods; however, these methods do not ensure the shelf-stability or safety of the products. Recently, the bio-preserver for packed bread SL778, containing L. plantarum CRL 778, was developed in our laboratory11. The commercialization of the SL778 starter needs to consider additional features such as those technological ones addressed in the present study. Indeed, the SL778 bio-preserver lifetime at 4 and −20°C was established and sensory attributes of the resulting breads were explored.
The characteristics of the SL778 starter were quite stable during 14-day storage at 4°C while at −20°C, cell viability and lactic acid concentration, showed a significant (p<0.05) decrease as from day 7. Gaggiano et al.7 reported that the microbial composition and acidifying activity of a similar multi-species semi-liquid starter were kept constant during 21 days of storage at 4°C. In addition, the storage of this multi-species starter for a period longer than 21 days (e.g., 30 days) caused a significant (p≤0.05) decrease in the acidification activity in all the assayed conditions.
Although SL778 maintained its efficacy on bread preservation until 14-day storage at both temperatures, the decrease in cell viability and lactic acid concentration of the starter at −20°C might affect the effectiveness of SL778 since the pH of the lowering dough during the fermentation step increase the undissociated fraction of the organic acids with antifungal activity, mainly CPa11. LAB acidification in wheat fermentation, in addition, have other beneficial properties, for example: (i) creates an optimum pH for the activity of endogenous proteinases22 which improves texture changes2 and flavor17 and (ii) delays starch retrogradation and bread firming3,12,13.
Although the influence of the semi-liquid starter was assayed, the impact of different conditions for the storage of the starter and their effect on the sensory properties of the breads was especially assessed. Despite the scarce discriminative capability of the panel (points slightly higher than 3, value corresponding to “indifferent”), little differences were perceived in breads containing SL778 with respect to the control bread only containing CPa, the panel observed greater sensitivity for acidic taste than for acidic smell. The higher amount of lactic acid and TTA found in the SL778 starter stored at 4°C was not detected in the sensoryl attributes of tested breads and no difference was indicated with respect to bread manufactured with SL778 stored at −20°C.
In European countries, the industries have shown an increasing interest in type II sourdoughs, which are semi-fluid preparations with lactic acid bacteria stored until use (up to one week), to be employed in the manufacture of a variety of products such as breads, cakes and crackers3,23. To our knowledge, only a multi-species semi-liquid sourdough starter containing three strong acidifying LAB strains, L. casei DPPMA27, L. fructivorans DPPMA8 and Weissella confusa DPPMA20, was scientifically developed7. In this study, the SL778 semi-liquid bio-preserver produced by fermentation without pH control can be stored at 4 or −20°C without modifying its antifungal activity during 14 days. It may represent a useful and accessible tool for getting regional baked goods with standardized quality, especially at the storage temperature of 4°C, since only a refrigerator is needed. Nevertheless, further trials have to be carried out to assess a longer storage time.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors acknowledge the financial support of CONICET (PIP0077), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PICTs 1124 and 1435) and Consejo de Investigación de la Universidad Nacional de Tucumán (CIUNT, 26/D427) from Argentina.