Scedosporium species are considered emerging agents causing illness in immunocompromised patients. In Chile, only Scedosporium apiospermum, Scedosporium boydii and Lomentospora prolificans haven been reported previously.
AimsThe study aimed to characterize genetically Scedosporium dehoogii strains from Chilean soil samples, and assessed the antifungal susceptibility profile to classic and novel putative antifungal molecules.
MethodsIn 2014, several samples were obtained during a survey of soil fungi in urban areas from Chile. Morphological and phylogenetic analyses of the internal transcribed spacer region (ITS), tubulin (TUB), and calmodulin (CAL) sequences were performed. In addition, the susceptibility profiles to classic antifungal and new putative antifungal molecules were determined.
ResultsFour strains of Scedosporium dehoogii were isolated from soil samples. The methodology confirmed the species (reported here as a new record for Chile). Antifungal susceptibility testing demonstrates the low activity of terpenes (α-pinene and geraniol) against this species. Voriconazole (VRC), posaconazole (PSC), and the hydroxyquinolines (clioquinol, and 5,7-dibromo-8-hydroxyquinoline) showed the best antifungal activity.
ConclusionsOur results demonstrate that Scedosporium dehoogii is present in soil samples from Chile. This study shows also that hydroxyquinolines have potential as putative antifungal molecules.
Las especies de Scedosporium se consideran agentes emergentes responsables de enfermedad en pacientes inmunodeficientes. En Chile, únicamente se había publicado con anterioridad la existencia de las especies Scedosporium apiospermum, Scedosporium boydii y Lomentospora prolificans.
ObjetivosEste estudio tuvo como objetivo clasificar genéticamente aislamientos de Scedosporium dehoogii obtenidos de muestras del suelo de Chile. Asimismo, se evaluó el perfil de sensibilidad de las cepas a los antifúngicos clásicos y a nuevas moléculas con potencial antifúngico.
MétodosEn el año 2014, durante un estudio de evaluación de la biodiversidad fúngica en Chile, se tomaron diversas muestras del suelo de zonas urbanas del país. Se llevaron a cabo análisis morfológicos y filogenéticos de las secuencias pertenecientes a la región del espaciador transcrito interno (ITS), de la tubulina (TUB) y de la calmodulina (CAL). Además, se determinaron los perfiles de sensibilidad a los antifúngicos clásicos y a nuevas moléculas con potencial antifúngico.
ResultadosSe aislaron cuatro cepas de Scedosporium dehoogii de las muestras del suelo. Las pruebas morfológicas y moleculares confirmaron la especie (el presente estudio representa un nuevo reporte para Chile). Las pruebas de sensibilidad antifúngica mostraron baja actividad de los terpenos (α-pineno y geraniol). El voriconazol (VRC), el posaconazol (PSC) y las hidroxiquinolinas (clioquinol y 5,7-dibromo-8-hidroxiquinolina) presentaron la mejor actividad antifúngica.
ConclusionesNuestros estudios demuestran que Scedosporium dehoogii está presente en los suelos de Chile. Asimismo, este estudio sugiere que las hidroxiquinolinas desempeñan una potencial actividad antifúngica.
Species of Scedosporium are ubiquitous fungi found in several substrates, such as compost, soil, sewage, and water. Some species can cause a wide spectrum of infections in humans and animals.17,24 Up to date, only five species have been related to human infections: Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium dehoogii, Scedosporium boydii, and Lomentospora prolificans (formerly Scedosporium prolificans).13,15,22,34 Scedosporiosis (mycosis caused by Scedosporium species)7,17 mostly affects immunocompromised patients, causing a wide range of infections as brain abscesses, lungs disease, meningitis, mycetoma, septic arthritis, osteomyelitis, etc.6,20,25–27 In Chile, only three clinical cases have been reported: a rhinosinusitis by S. boydii,31 a surgical site abscess by L. prolificans10 and a rhinosinusal infection by S. apiospermum.3
The treatment of scedosporiosis is still a challenge due to the high mortality rates and the increasing frequency.37 In addition, several studies have reported that Scedosporium species show multiple in vitro resistance patterns to most antifungals, with high intraspecific variability.14,17,23,30 On the other hand, voriconazole (VRC) remains as the most effective antifungal agent. However, several species show high minimum inhibitory concentrations (MICs) values to VRC, as S. dehoogii (up to >16μg/ml) or L. prolificans (geometric mean MIC of 15.4μg/ml).23
Currently, the number of antifungal drugs available for the treatment of fungal infections is still limited. Likewise, the increasing frequency of resistance makes the development of new therapeutic strategies necessary. In this way, several authors have reported the potential of natural compounds isolated from plants as antifungal agents.18,29,35 In this regard, some compounds such as terpenes (geraniol, carvacrol, α and β pinene) and quinolines (hydroxyquinolines) have been proposed as good candidates with biocide activity.1,9,12,33
In the present work, we report the isolation of four strains of S. dehoogii from Chilean soil samples. Our searching in the literature showed no previous findings of S. dehoogii in Chile. In addition, we have performed antifungal assays in order to determine the susceptibility patterns to classic antifungal agents and new putative antifungal molecules.
Materials and methodsFungal strainsA total of 84 soil samples were collected from 14 administrative regions. At each sampling site, triplicates were obtained and placed in sterilized polyethylene bags. Then, 1ml of each soil suspension (1:10 w/v dilutions) was cultured on the selective medium Dichloran Rose-Bengal Chloramphenicol Agar (DRBC) (Becton & Dickinson). The DRBC plates were stored at 25°C and 37°C, and observed every two days. When typical features of Scedosporium species were observed, the colonies were subcultured in 2% malt extract agar (MEA), potato dextrose agar (PDA) and Sabouraud agar (SA), at 25°C and 37±1°C in the dark. The strains studied in the present work were isolated from soil collected in urban areas from several Chilean cities: one from Arica (North of Chile); two from Valparaiso (central region of Chile) and the last from Puerto Varas (South of Chile).
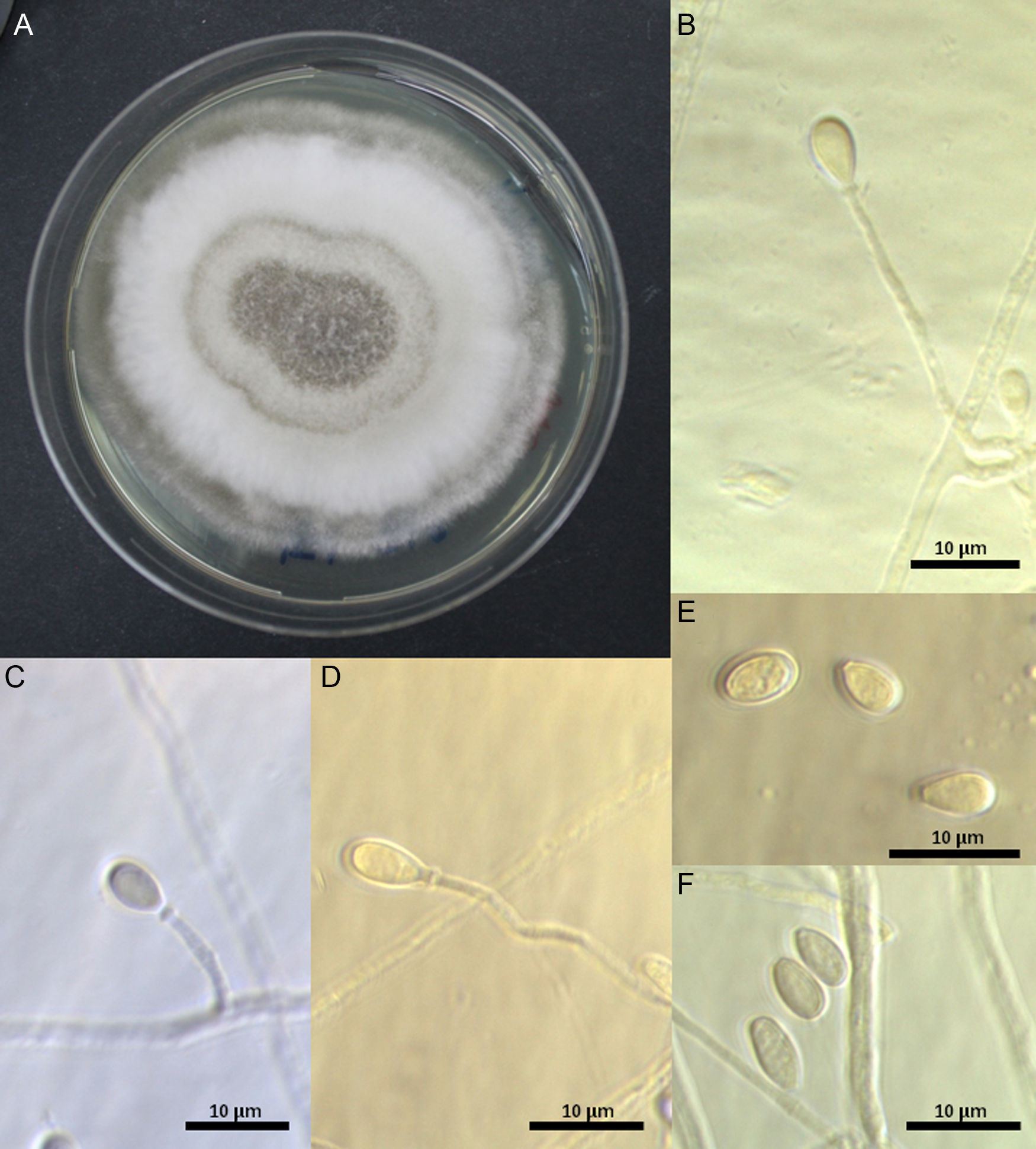
Morphological and physiological studyMorphological features were determined on PDA at 25±1°C (7 days). Cultures were deposited at the Chilean Fungal Collection (ChFC), Mycology Unit, University of Chile as S. dehoogii ChFC 148, 151, 153, and 161. Microscopic features were studied in lactophenol stained with cotton blue (Merck) using Optika B1000Ph microscope (Optika, Italy). Microphotographs were taken with Optikam B9 camera (Optika, Italy).
DNA extraction, amplification, and sequencingFungal DNA was extracted directly from pure 7 day-old cultures by means of the E.Z.N.A® Fungal DNA Mini Kit (Omega Biotek Store, USA). PCR was conducted with a 3Prime Thermal Cycler (Techne, UK) using ITS1 and ITS4 primers for ITS1-5.8 S-ITS2 region36; Cal1 and Cal2 primers for the calmodulin gene,28 and Tub2F and Tub2R primers for one region within the β-tubulin gene.8 A 25μl-mixture containing 17μl of BioMix™ Red (New England Biolabs), 10pM of the primers, and 10ng DNA was amplified following a protocol of initial denaturation at 94°C for 5min, 35 cycles of denaturation at 94°C for 1min, annealing at 52°C for 1min for ITS region, and 55°C for CAL and TUB; extension at 72°C for 2min, and final extension at 72°C for 7min. The PCR product was checked on 1% agarose gel. Purification was performed with FavorPrep™ Gel/PCR purification Mini Kit (Favorgene, Taiwan) following the manufacturer guidelines. Sequencing was done on Macrogen (Macrogen, Korea). An NCBI Blastn search for each locus region was performed.2 Sequences from type and reference strains were gotten from GenBank database. The sequences were aligned in MUSCLE software11 followed by manual adjustments with a text editor.
Phylogenetic analysesPhylogenetic analyses were performed using Maximum Parsimony (MP) method in PAUP 4.0b10 software. For MP inference the most-parsimonious tree (MPT) was obtained after one hundred heuristic searches with random sequence addition and tree bisection-reconnection branch-swapping algorithms, collapsing zero-length branches and saving all minimal-length trees (MulTrees). Support for internal branches was assessed using a heuristic parsimony search of 1000 bootstrapped data sets. The combined datasets of the ITS, CAL, and TUB were tested for incongruence with the partition homogeneity test and implemented in PAUP 4.0b10 software. Lomentospora prolificans FMR 7294 and Petriellopsis africana CBS 311.72 were used as outgroup.
Antifungal susceptibility testingIn vitro susceptibility testing was performed using broth microdilution for filamentous fungi according to CLSI document M38-A2.5 The MICs and MECs (minimal effective concentration) were read at 48 and 72h. Although the clinical breakpoints for Scedosporium species have not been developed, the following suggested cut-off breakpoints were used: susceptible (S), ≤1μg/ml; intermediate (I), 2μg/ml; and resistant (R), ≥4μg/ml (CLSI, M38 A-2). Pure active powders, of known potency, of amphotericin B (AMB) (Sigma–Aldrich), voriconazole (VRC) (Sigma–Aldrich), itraconazole (ITC) (Sigma–Aldrich), posaconazole (PSC) (Sigma–Aldrich), caspofungin (CSF) (Sigma–Aldrich), micafungin (MCF) (Astellas Pharma), anidulafungin (ANF) (Pfizer), α-pinene (α-P) (partner laboratory), geraniol (GER) (partner laboratory), clioquinol (CLIO) (Sigma–Aldrich), and 5,7-dibromo-8-hydroxyquinoline (5–7C) (Sigma–Aldrich) were used. All tests were performed in duplicates. Candida parapsilosis ATCC 22019 and Paecilomyces variotii ATCC MYA 3630 were included as quality controls.
ResultsMorphology and physiologySeveral genera and species were isolated from soil samples (i.e., Aspergillus, Cunninghamella). In the same way, other Scedosporium and related species such as S. boydii, S. apiospermum, and L. prolificans were isolated on DRBC plates (unpublished data).
In the first approach, all Scedosporium isolates were identified based on the morphological and physiological features. The final identification for each species recovered from DRBC plates was obtained by molecular analyses of tubulin gene sequences.
In general, the four strains studied displayed the typical features described by Gilgado et al.15 The colonies on PDA reached 28mm diameter after 7 days at 25±0.2°C, they were dense, cottony to lanose, grayish-white to white, and with a whitish irregular margin; the reverse was yellowish or light-brown. Solitary conidiogenous cells were subhyaline smooth-walled, mainly cylindrical, 10–54μm length by 1–1.4μm width, and produced pale brown, obovoid or ellipsoidal 5.9–6.2μm×3.6–4.4μm conidia. Sessile conidia were subhyaline, thin-walled, mostly obovate (5.9−)7.3(−7.8)×(3.5−)3.8(−4.0)μm. Hyphae, 1.2–2.5μm, were hyaline to subhyaline (Fig. 1). A teleomorph was not observed even after 60 days of incubation.
The growth rates were similar in all media tested. The minimum, maximum and optimal temperatures were 15°C, 40°C and 36±1°C respectively.
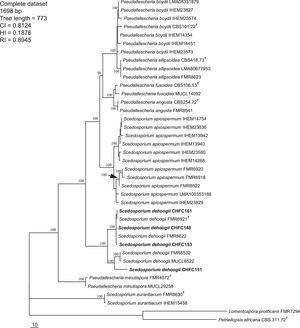
PhylogenyThe identification of all the strains studied as S. dehoogii was feasible by means of the three loci used in the present study. The partition homogeneity test demonstrated that the three loci sequence data sets were congruent (p=0.05) and could therefore be combined. A fragment of 1698bp was obtained in the concatenated analysis. The phylogeny inference was analyzed as separate (data not shown) and combined dataset.
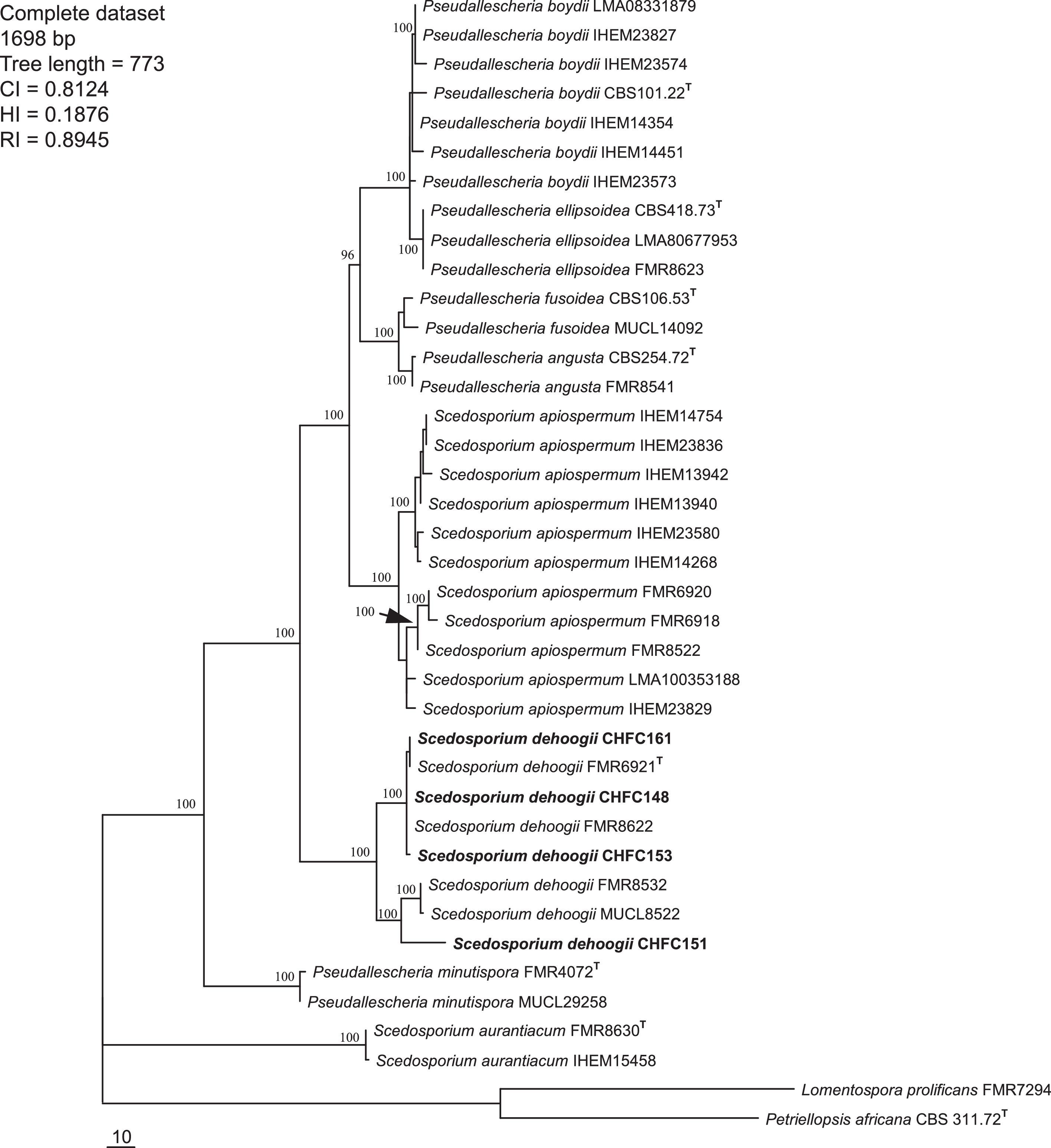
The tree topologies of concatenated loci by MP were highly similar to those observed in the individual tree for each gene tested. High clade support values were obtained in the concatenated analysis, being mainly over 90%. A total of 90 MPT were obtained from heuristic search from concatenated dataset (Fig. 2). The trees had a consistency index of 0.81, a retention index of 0.89, and a homoplasy index of 0.18. Analyses of the combined partitions allowed the identification and recognition of 8 species of Scedosporium (Fig. 2). In the same way, the separation of L. prolificans from the Scedosporium clade was observed. The four strains isolated from Chilean soil samples were placed at the S. dehoogii clade, with the reference and type strains sequences.
One of the 90 most-parsimonious trees obtained from heuristic searches based on the analysis of the combined data set (ITS+CAL+TUB). Bootstrap support values of 100% are indicated at the nodes. CI, consistency index; RI, retention index; HI, homoplasy index. Chilean strains are indicated with boldface type. T=type strains.
The sequences generated in this study were deposited in the GenBank database under the following accession numbers: KT600015 (ChFC148 ITS), KT600016 (ChFC151 ITS), KT600017 (ChFC153 ITS), KT600018 (ChFC161 ITS), KT820715 (ChFC148 TUB), KT820716 (ChFC151 TUB), KT820717 (ChFC153 TUB), KT820718 (ChFC161 TUB), KT820719 (ChFC148 CAL), KT820720 (ChFC151 CAL), KT820721 (ChFC153 CAL), KT820722 (ChFC161 CAL).
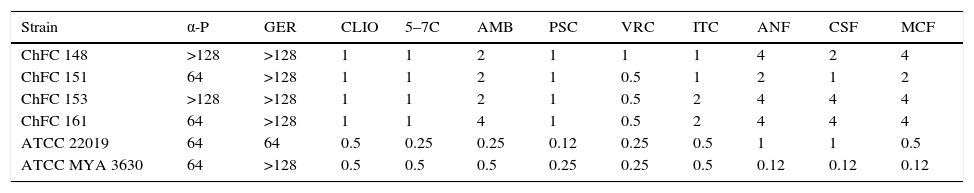
Antifungal susceptibility testingThe results of the antifungal susceptibility tests for the Scedosporium strains are shown in Table 1. Among the classical antifungal agents, voriconazole, itraconazole and posaconazole were the most active. Echinocandins were the less active against all the strains. Among the putative new antifungal molecules, geraniol and α-pinene were not active, obtaining high MIC values. On the other hand, clioquinol, and 5,7-dibromo-8-hydroxyquinoline showed promising results with low MICs values.
In vitro activities of classical antifungal agents and novel putative antifungal compounds against four isolates of Scedosporium dehoogii. MIC (μg/ml) values MEC (μg/ml) values.
| Strain | α-P | GER | CLIO | 5–7C | AMB | PSC | VRC | ITC | ANF | CSF | MCF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ChFC 148 | >128 | >128 | 1 | 1 | 2 | 1 | 1 | 1 | 4 | 2 | 4 |
| ChFC 151 | 64 | >128 | 1 | 1 | 2 | 1 | 0.5 | 1 | 2 | 1 | 2 |
| ChFC 153 | >128 | >128 | 1 | 1 | 2 | 1 | 0.5 | 2 | 4 | 4 | 4 |
| ChFC 161 | 64 | >128 | 1 | 1 | 4 | 1 | 0.5 | 2 | 4 | 4 | 4 |
| ATCC 22019 | 64 | 64 | 0.5 | 0.25 | 0.25 | 0.12 | 0.25 | 0.5 | 1 | 1 | 0.5 |
| ATCC MYA 3630 | 64 | >128 | 0.5 | 0.5 | 0.5 | 0.25 | 0.25 | 0.5 | 0.12 | 0.12 | 0.12 |
AMB, amphotericin B; VRC, voriconazole; ITC, itraconazole; PSC, posaconazole; CSF, caspofungin; MCF, micafungin; ANF, anidulafungin; α-P, α-pinene; GER, geraniol; CLIO, clioquinol; 5–7C, 5,7-dibromo-8-hydroxyquinoline; ChFC, Chilean Fungal Collection; ATCC, American type culture collection.
Scedosporium species are opportunistic pathogenic fungi. Several species have been reported as etiological agents, especially in immunocompromised patients.
S. dehoogii is considered an environmental species. In recent studies, several strains were isolated from locations associated with high human activity: playgrounds, riverbanks or agricultural areas (except vineyards).19,21,32 Thus, S. dehoogii is not commonly associated with human infections, despite the high virulence observed in murine models.16 However, some clinical isolates have been recovered: one from a sympathetic ophthalmia in a 10 year-old boy,4 one from cystic fibrosis sputum, and two from cutaneous infections.23
In Chile, few cases of scedosporiosis have been reported.3,10,31 These three cases described previously, with the identification based on the morphological features of the isolates, were infections by S. apiospermum, S. boydii and L. prolificans, the most prevalent agents described on the literature. In the present study, we report for the first time the presence of S. dehoogii in soil samples of our country. The preliminary morphological identification was confirmed using the molecular tools, agreeing with the information previously reported by other authors.16 In this way, four strains isolated from Chile were located in the S. dehoogii clade. However, some variability was observed in their nucleotide sequences, confirming the observations of Gilgado et al.13
Although data of the in vitro susceptibility of this fungus is limited, high MECs for echinocandins have been described in previous studies,14,23 which is in agreement with our results. In the present study VRC and PSC were the most active drugs, being within the range of susceptibility. This finding agrees with the results reported by Gilgado et al.14 However, other authors have found that S. dehoogii is associated with high VRC MICs.23 This could be explained by the fact that these authors tested a greater number of isolates.
Clioquinol and 5,7-dibromo-8-hydroxyquinoline seem to be putative antifungal agents as they showed a good activity against the four strains, having similar MIC values to those obtained against some azoles tested here.
In summary, we have reported for the first time the presence of S. dehoogii in Chile, and its susceptibility against classical and novel putative antifungal drugs. More studies are necessary to evaluate the spectrum of all the Scedosporium species present in Chile. In addition, more in vitro and in vivo analyses with a large number of strains are necessary to elucidate the potential activity of clioquinol, 5,7-dibromo-8-hydroxyquinoline and other hydroxyquinolines as antifungal agents.
Conflict of interestThe authors declare no conflict of interest.
This work was supported by the FONDECYT project 11130381 and U-INICIA 560786.
The authors thank Dr. Valentina Salas for the reviewing of the manuscript and constructive comments. Authors also thank Felipe Salas for some soil samples collecting and geraniol and α-pinene samples. We thank Armin Araya for the Arica soil samples.