Therapeutic goals in inflammatory bowel disease (IBD) have evolved with the introduction of biologic therapies. These medications have demonstrated that resolution of mucosal inflammation was feasible. Mucosal healing has been associated with fewer complications and better patient outcomes. Hence, symptomatic control, which was considered the primary treatment goal, is no longer sufficient. Mucosal healing is now the principal target. Several biomarkers of inflammation have been studied, including C-reactive protein and fecal calprotectin. Although they are helpful in monitoring disease activity, they are still not considered therapeutic targets at this time. Ongoing research is evaluating new biomarkers as potential future targets. Resolution of histological inflammation has also been associated with better outcomes, however, the evidence is limited and the definition of histologic healing is still not clear. Ultimately, restoring quality of life is essential. In recognition of the patient's goals in wellness, patient reported outcomes are part of the therapeutic goals. When combined with mucosal healing endpoints, patient reported outcomes serve as a composite endpoint in IBD clinical trials and now patient care.
La introducción de terapias biológicas para el tratamiento de la Enfermedad Inflamatoria Intestinal (EII) demostró que la resolución de la inflamación mucosa era factible. Subsecuentemente, se ha demostrado que la curación de la mucosa se asocia a remisión clínica prolongada, menor tasa de cirugías, menor tasa de hospitalizaciones y mejor calidad de vida. Por lo tanto, el control sintomático que hasta entonces se consideraba como objetivo terapéutico principal ya se no considera suficiente. Estudios recientes han demostrado que la resolución de la inflamación histológica también se ha asociado con mejores resultados, sin embargo, la evidencia es limitada y la definición de curación histológica aún no está clara. En adición, múltiples biomarcadores han sido estudiados con el fin de encontrar un método alternativo a la endoscopía, entre ellos la proteína C reactiva y la calprotectina fecal. Sin embargo, aunque éstos son útiles para monitorear la actividad de la enfermedad, no son considerados objetivos terapéuticos. Nuevos biomarcadores están bajo investigación. A pesar de que la resolución sintomática no es considerada como el objetivo terapéutico principal, el reconocimiento de la percepción del paciente respecto a su enfermedad, mejor conocido como resultados comunicados por el paciente, forman parte de los objetivos terapéuticos junto con la curación mucosa.
The use of anti-tumor necrosis factor (anti-TNF) therapies in the management of inflammatory bowel disease (IBD) has redefined treatment goals. Before these medications were introduced treatment was guided on clinical parameters and symptomatic control.1 However, anti-TNFs were able to demonstrate that healing the intestinal lining was possible,2 and although mucosal healing can be achieved with several medications including corticosteroids, mesalamines and immunomodulators, data suggests that biologics may be more robust.1 Several studies have shown that mucosal healing (MH) is associated with long term corticosteroid-free clinical remission use and protection from colectomy in ulcerative colitis,3 hence treatment goals have now evolved to include MH as a target. Recently, the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) initiated the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) program whose objective it was to garner international expert consensus on evidence-based treatment targets for IBD.4 The objective of this article is to review and discuss what has been proposed as therapeutic targets in IBD.
2Therapeutic targets2.1SymptomsOver the years, different scoring systems have been developed and used to assess for disease activity in both ulcerative colitis (UC) and Crohńs disease (CD). The Mayo Score (MS) was described by Schroeder et al. in 1987 and has since become one of the most commonly used scoring systems in UC.5 The Mayo score does have an endoscopic component (Table 1). The most frequently used scoring systems in CD are the Crohńs Disease Activity Index (CDAI) which was described by Best at al. in 1976 6and the Harvey Bradshaw Index (HBI) which was created by Harvey and Bradshaw in 1980.7
Mayo Score.
| Parameters | Subscores 0-3 |
|---|---|
| Stool frequency | 0=normal number of stools for the patient1=1-2 stools more than normal2=3-4 stools more than normal3=5 or more stools more than normal |
| Rectal bleeding | 0=no bleeding1=streaks of blood with stool less than half of the time2=obvious blood with stool most of the time3=blood alone passed |
| Findings of flexible proctosigmoidoscopy | 0=normal or inactive1=mild disease (erythema, decreased vascular pattern, mild friability)2=moderate disease (marked erythema, absent vascular pattern, friability, erosions)3=severe disease (spontaneous bleeding, ulceration) |
| Physician's global assessment | 0=normal1=mild disease2=moderate disease3=severe disease |
Reference: Schroeder KW, Tremaine WJ, IIstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–9.
For UC, clinical remission has been defined as a total MS ≤2, with no subscore>1; in CD clinical remission has been defined as a CDAI score<150 or HBI score of ≤4 points.8,9 On the other hand, clinical response has been defined as a reduction in the MS of ≥3 points and ≥30% from baseline, with a decrease in the rectal bleeding subscore of ≥1 point or a subscore of ≤1 for a patient with UC.10,11 In CD, the definition for clinical response has varied by clinical trials. Some of the definitions proposed are ≥ 70-point decrease in CDAI,8,12 ≥100-point decrease in CDAI(8,12), or a ≥70-point decrease in CDAI plus a ≥25% baseline reduction in score.13 In regards to HBI, Vermeire et al conducted a study that determined that CDAI and HBI were positively correlated and concluded that clinical response was defined as a 3 point decrease in the HBI score.9
However, some studies have shown that symptoms do not correlate with mucosal activity, particularly in patients with CD.14 For example, in the SONIC trial which included patients with moderately active CD (CDAI score>220), 20% of the patients did not have ulcerations on ileocolonoscopy at baseline.15 This discrepancy between symptoms and absence of mucosal inflammation has several potential explanations. One explanation could be persistent histological inflammation in the setting of endoscopic remission causing persistent symptoms.16,17 Another possibility is the presence of irritable bowel syndrome like symptoms.18–20 Vivinus-Nébot et al.20 have proposed that the presence of IBS-like symptoms is related to persistent subclinical and ongoing inflammation which is associated with increased colonic paracellular permeability. In addition, chronic inflammation in both UC and CD may lead to significant structural damage of the bowel wall, which can manifest itself as decreased motility, narrowing of the colon and rectum and changes in the absorptive function17 Furthermore, inflammation may also produce direct damage to the enteric nervous system and alter motility.21
In addition, the CDAI score has several limitations. It is subjective as it assesses the patient's perception of their disease; it is difficult to obtain as the score is based on a diary which is completed by the patient for 7 days prior to evaluation, which precludes its use in clinical practice and also has interobserver variability. Furthermore, it is not accurate on patient with stenotic or fistulizing behavior.22
Therefore, although assessment of clinical parameters is still necessary, resolution of symptoms alone is not a sufficient target.4 Given that for now our therapies are directed at inflammatory pathways, patients are unlikely to respond to any current therapy if there is no evidence of intestinal inflammation. Patients with symptoms and no mucosal inflammation or persistent symptoms after resolution of intestinal inflammation must be evaluated for structural damage and other causes of symptoms such as bacterial overgrowth, irritable bowel syndrome, and bile salt diarrhea.
2.2Mucosal healingOver the last few decades it has been recognized that the best outcomes have been observed in patients who achieve mucosal healing. Historically, endoscopic disease activity has been assessed by different scores in UC and CD.
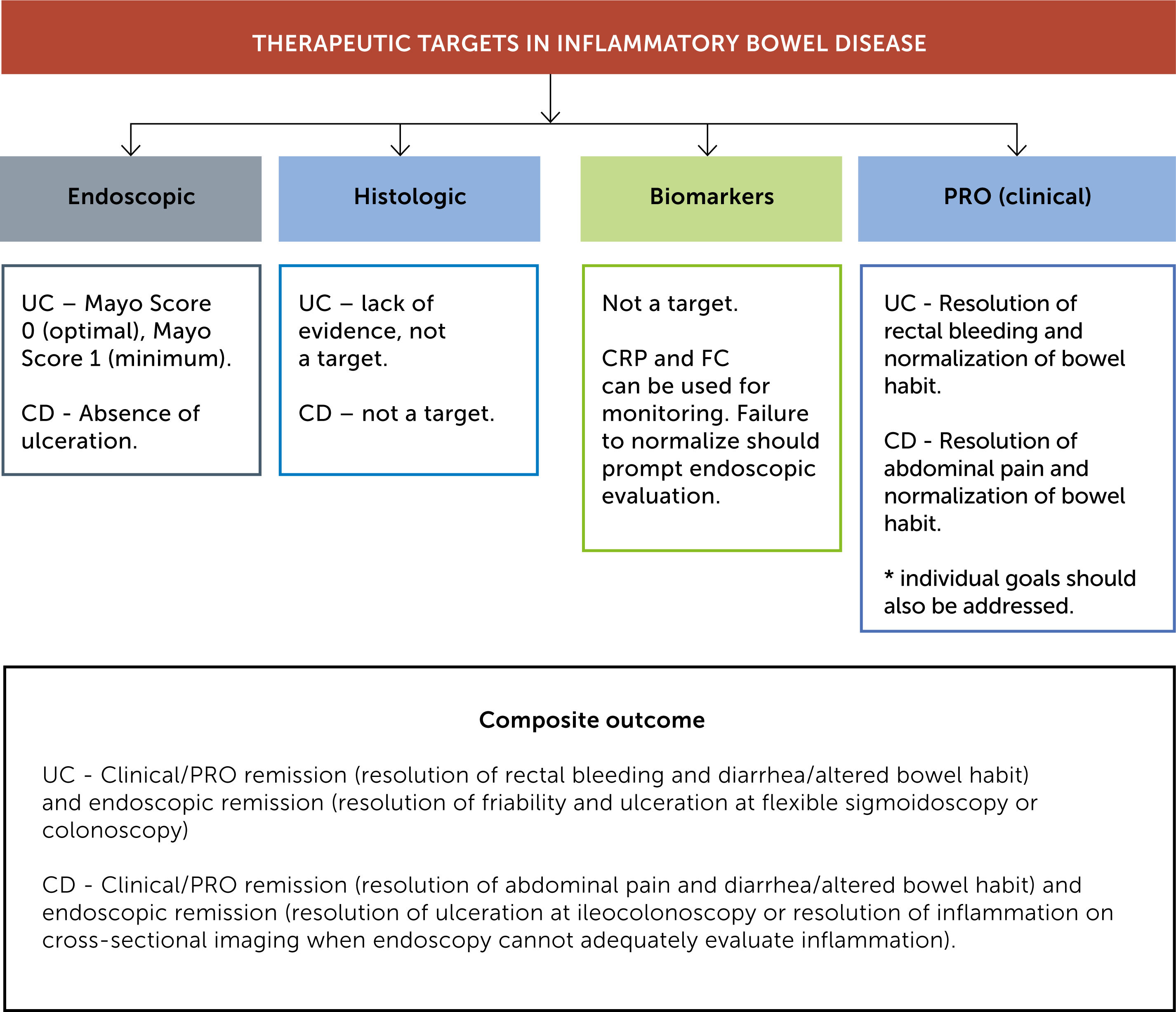
In UC, the STRIDE program group recommends the use of endoscopic Mayo score rather than the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) given its simplicity and well-established predictive value.4 The Mayo scoring system goes from 0-3; 0 meaning there is no disease activity and the mucosa is normal; 1 mild disease (erythema, decreased vascular pattern and mild friability); 2 moderate disease (marked erythema, absent vascular pattern, friability, erosions); 3 severe disease (ulceration, spontaneous bleeding).5 Although achieving an endoscopic Mayo score of 0 sounds ideal, there is insufficient evidence to recommend this as a target for all patients but an endoscopic Mayo score of 1 should be the minimal target. Experts have recommended to assess endoscopic disease activity 3-6 months after the patient has been started on therapy and to consider an endoscopic Mayo score of 0-1 as endoscopic remission.4
For CD, 2 different validated scoring systems have been used in clinical trials to assess disease severity, the CD Endoscopic Index of Severity (CDEIS) and the Simple Endoscopic Score for CD (SES-CD). The CDEIS score divides the bowel in 5 segments (rectum, sigmoid and left colon, transverse colon, right colon and ileum), assesses the presence of deep and superficial ulceration in each segment plus the extent of surface area involved and/or ulcerated (Table 2).23 The SES-CD was later developed and validated as a simpler scoring system by Daperno et al. This scoring system divides the bowel in the same 5 segments and ulcer size, the extent of ulcerated and affected surfaces, and the presence of stenosis are scored from 0-3 (Table 3).24 Although endoscopic remission has been commonly defined as a CDEIS<3 and SES-CD ≤2, currently there is no cutoff value to define MH and hence MH has been defined as absence of ulceration.4,14 In the case of CD, endoscopic remission should be assessed 6-9 months after starting a new therapy.4 For those patients in whom endoscopic assessment cannot be performed or is not informative, cross sectional imaging should be obtained.4
CDEIS.
| Ileum | Right Colon | Transverse | Left/Sigmoid Colon | Rectum | Sum | |
|---|---|---|---|---|---|---|
| Deep ulceration (0 points if none, 12 points if present) | Total 1 | |||||
| Superficial ulceration (0 points if none, 6 points if present) | Total 2 | |||||
| Surface involved by disease in cm (0-10 points)* | Total 3 | |||||
| Surface involved by ulceration in cm (0-10 points)* | Total 4 |
Total 1+Total 2+Total 3+Total 4=Total A Numbers (n) of segments totally or partially explored (1-5)=n Total A divided by n=Total B Quote 3 if ulcerated stenosis anywhere, 0 if not=Total C Quote 3 if non-ulcerated stenosis anywhere, 0 if not=Total D Total B+C+D=CDEIS
The 10cm scale represents the surface effectively explored. Reference: Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30(7):983–9.
SES-CD.
| Variable | Simple Endoscopic Score for Crohńs Disease values | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Size of ulcers | None | Aphthous ulcer (diameter 0.1-0.5 cm) | Large ulcer (diameter 0.5-2 cm) | Very large ulcer (diameter>2 cm) |
| Ulcerated surface | None | <10% | 10-30% | >30% |
| Affected surface | Unaffected segment | <50% | 50-75% | >75% |
| Presence of narrowings | None | Single, can be passed | Multiple, can be passed | Cannot be passed |
Total SES-CD=sum of the 4 variables in the 5 bowel segments (rectum, sigmoid/left colon, transverse colon, right colon and ileum).
Reference: Daperno M, D’Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60(4):505-12.
Several studies have addressed long-term outcomes of patient who achieve MH in UC and CD patients. A recent meta-analysis assessing the long-term effect of mucosal healing in patients with UC showed that MH was associated with higher rates of clinical remission, colectomy avoidance, sustained MH, and corticosteroid-free clinical remission.3 A meta-analysis in patients with CD showed that patients who achieved MH had higher rates of achieving long-term clinical remission, long-term MH, and a trend towards decreased rate of CD-related surgeries in comparison to those patients with active CD who did not achieve MH.25
2.3ImagingImaging techniques are a good alternative for those patients in which endoscopy cannot be performed or is not informative. Several imaging techniques are available, including ultrasonography, computed tomography (CT) and Magnetic Resonance Imaging (MRI). The latter has the advantage of avoiding the use of ionizing radiation and hence should be preferred over CT imaging specially in patient with CD who will undergo repeated examination due to the natural history of the disease.26 However, magnetic resonance enterography interpretation requires training; data suggests that diagnostic accuracy is accomplished when feedback is provided on 100 cases.22 On the contrary, cross-sectional imaging techniques in UC have a limited role.4
The most common used score is the Magnetic Resonance Index of Activity (MaRIA) which takes into account bowel wall thickness (mm), presence of mucosal ulceration, presence of mural edema, presence of pseudopolyps in the lumen, enlarged (>1cm) regional mesenteric lymph nodes, quantitative measurement of wall signal intensity and relative contrast enhancement of the intestinal wall in six different segments (distal ileum, ascending, transverse, descending, sigmoid colon and rectum).27 This score has shown to be highly correlated with CDEIS. Some years later a similar index was created which used diffusion-weighted imaging (DWI) sequence instead of contrast enhancement called DWI-MaRIA.26
On the other hand, MRI is also useful in detecting disease related complications such as fistulas. The Van Assche index is the most frequently used index for perianal disease.22 The score takes into account the number of fistula tracts, location, extension, hyperintensity on T2 weight images, collections, rectal wall involvement.28 This index has been partially validated.
Ultrasonography (US) is another accessible, non-invasive, inexpensive technique that can be used in the evaluation of IBD.29 This technique can assess bowel wall thickening, pseudostratification, inflammatory mass, loss of colonic haustration as well as complications such as strictures, abscesses and fistulas.29 In addition, the use of doppler US can be used to evaluate bowel wall vascularity, as increased vascularity is seen in inflammatory processes.22,29 The use of oral and/or intravenous contrast can help increase diagnostic accuracy and improve sensitivity.22,29 However, to date, there are no validated indexes for luminal activity based on US.22
The STRIDE program recommends to assess resolution of inflammation by cross-sectional imaging as target in patient with CD when endoscopy cannot evaluate inflammation adequately. In patients with UC, cross-sectional imaging is not a target.4
2.4Histological healingSeveral studies have assessed histological healing in patients with UC and have shown that persistent histologic inflammation have been associated with risk of relapse, hospitalization and surgery.16,30–34 The Geboes score and the Riley score are the most commonly used scores in UC.22 The Riley score was described by Riley at al.16 in 1991 and evaluates for acute inflammatory cell infiltrate, crypt abscesses, mucin depletion, surface epithelial integrity, chronic inflammatory cell infiltrate and crypt architectural irregularities. Grades range between none, mild, moderate, or severe, which makes it difficult to reproduce. The Geboes score was later created and evaluates architectural changes, chronic inflammatory infiltration, lamina propria neutrophils and eosinophils, neutrophils in epithelium, crypt destruction, and erosions or ulcerations.35 Active histology is defined as Geboes score ≥3.1 (presence of neutrophils in the epithelium).32 Basal plasmacytosis, defined as plasma cell infiltrate in the lower third of the lamina propria, has also been shown to be an independent factor of relapse in UC.34
Most recently Mosli et al.36 developed and alternative instrument called the Robarts Histopathology Index due to the fact that the previous scores mentioned lacked formal assessment of index reliability, responsiveness and validity. In their study biopsies were scored using the Geboes score, modified Riley score and a visual analogue scale global rating. Analysis of intra-rater and inter-rater reliability for each index and individual index items was performed. Only items with high reliability were used to develop the Robarts Histopathology Index which included: chronic inflammatory infiltrate, lamina propria neutrophils, neutrophils in the epithelium and erosion or ulceration. Total score ranges from 0 (no disease activity) to 33 (severe disease activity). However, the prognostic value of Robarts Histopathology Index has not been assessed and hence prospective cohort studies are needed in order to define potential application of the index.36
On the other hand, in patients with CD, discontinuous and transmural inflammation may induce sampling error for histological assessment and hence histological healing will not necessarily represent quiescent disease.37 The Colonic and Ileal Global Histologic Disease Activity Score is one of the few scores described and the most widely used.22 The score assesses epithelial damage, architectural changes, mononuclear or polymorphonuclear cells in the lamina propria and epithelium, presence of erosions/ulcers and granulomata, as well as the number of segmental biopsy specimens affected.37 The score is not validated and its role is not yet defined.37
To date there is no clear definition for histological healing or consensus in which histologic score should be used,14 hence histological healing is currently not recommended as a target in both clinical practice or clinical trials.4,14
2.5BiomarkersThe identification and utilization of noninvasive biomarkers has become of great interest over the last decades as they might be able to assist in the monitoring of mucosal inflammation in patients with IBD and potentially reduce the use of colonoscopy. To date, the most commonly used tests are C-reactive protein (CRP) and fecal calprotectin (FC). CRP is a widely available and inexpensive tool in the management of IBD, however its production is enhanced in a variety of systemic inflammatory diseases and is not exclusive to intestinal inflammation.38
CRP lacks sensitivity. In a prospective study, 71% and 25% of patients with UC and Crohn's disease, respectively, had normal CRP levels at diagnosis.39 Active ileal disease in particular can be associated with a normal CRP.40 In addition, a CRP+1059 G/C polymorphism in CRP gene was found to be associated with decreased serum CRP levels in patient with CD.41 In this study, homozygous and heterozygous carriers of the CRP+1059G/C polymorphism had lower mean serum CRP levels compared to wildtype carriers. The genotype frequencies were noted to be 0.1%, 8.4% and 91.5% respectively for homozygous, heterozygous and wildtype carriers in the single nucleotide polymorphism database of the National Center of Biotechnology Information (NCBI), which has the largest population data on the+1059 G/C polymorphism (1016 individuals).41
On the other hand, FC has shown to be one of the most reliable, non-invasive diagnostic tools for management of IBD.42 Calprotectin is a calcium and zinc binding protein considered to be neutrophil-specific, and accounts for around 60% of total soluble proteins in the cytoplasm of neutrophils.43 Therefore, the amount of calprotectin in stool is proportional to the neutrophil migration to the gastrointestinal mucosa.44 A recent meta-analysis showed that FC is a highly sensitive tool for assessing endoscopic activity with a pooled sensitivity of 85% and specificity of 75%.45
D’Haens et al. proposed a level of 250μg/g as cutoff to predict mucosal inflammation in both CD and UC. In their study, they found a positive predictive value (PPV) 48.5% and negative predictive value (NPV) of 96.6% in predicting endoscopic remission in patients with CD (CDEIS<3) and a PPV of 100.0% and NPV of 47.1% for active mucosal disease activity in patients with UC (Mayo>0).46 Fecal calprotectin is best used as a test for following individual patient's response to therapy rather than across populations of patients.
New noninvasive tests are being developed. One such test, Monitr, is a serum test that assesses MH by evaluating levels of 13 biomarkers associated with angiogenesis, cell adhesion, cell proliferation and repair, extracellular matrix remodeling, inflammation and immune cell recruitment in patients with CD.2 The testing provides a MH index score that reflects disease severity. Validation of the test showed an overall accuracy of 90%, with a negative predictive value of 92% and a positive predictive value of 87% for identifying patients with endoscopic activity.2
Currently, biomarkers are not considered a target for treatment per se, but are considered helpful tools in monitoring disease. CRP and FC may reflect residual intestinal inflammation, hence objective evaluation of the mucosa with endoscopy should be pursued if values fail to normalize.4
3Patient reported outcomes (PROs)Patient-reported outcomes (PROs) are self-administered tools that come directly from the patient without being interpreted by a physician or anyone else.47 PROs are now becoming important endpoints in clinical trials,48 as approval of IBD pharmacotherapy by the United States Food and Drug administration (FDA) now requires evaluation of PROs and objective measures of disease.14,47 To date, there are no PRO instruments created under the guidance of the FDA,49 however interim PROs derived from the CDAI and Mayo score have been created 4and have been included in the 12 recommendations to treat to target by the STRIDE group.4 The primary PRO for UC has been defined as resolution of rectal bleeding and normalization of bowel habits while primary PRO for CD has been defined as abdominal pain resolution and normalization of bowel habits; in addition, patient's individual goals should also be assessed.4 The STRIDE group recommends to assess PROs every 3 months until symptom resolution and every 6–12 months thereafter.
3.1Composite end-pointThe STRIDE program has recommended to achieve a composite target of clinical/PRO remission and endoscopic remission in both UC and CD. Recommendations made by the STRIDE program are summarized in Figure 1.
4ConclusionTherapeutic targets have evolved from clinical response to a composite end point including MH and PRO remission. Therapeutic interventions should be guided by periodic assessment of MH/PRO. Biomarkers are useful in monitoring the disease but are not a target for treatment per se. Although HH is associated with improved outcomes in UC, there is not consensus on best index and best way to minimize inter-observer variability. Ongoing research on new biomarkers and assessment of HH might change treatment paradigms in the future.
5Declaración conflicto de interesesDr. Daniela Fluxa has no disclosures.
Dr Abreu is a member of the scientific advisory boards of AbbVie Laboratories, Celgene Corporation, Shire Pharmaceuticals, Roche Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, AMGEN and GILEAD. She is on the advisory boards of Allergan, SERES and Nestle Health Science. She is a consultant for Prometheus Laboratories; Takeda; UCB, Inc.; Pfizer; Janssen and Eli Lilly Pharmaceuticals, Inc. She has performed training/consulting for Focus Medical Communications. She has performed lecturing/teaching for Imedex, Inc. She has lectured for CME Outfitters.