Training through medical simulation allows for continuous learning under controlled conditions. Simulation-based training activities can be used simultaneously with other educational strategies to strengthen the attitudinal skills needed to develop an informed consent process in the context of health research.
ObjectiveTo facilitate learning in undergraduate medicine students, and to evaluate their competences to carry out an informed consent process in a scenario that resembles reality.
Materials and methodsIn this semi-longitudinal study, a simulation-based activity was conducted with 136 medical students of the fourth (Group A) and fifth year (Group B) of the Marist University of Mérida, in southern Mexico.
ResultsThe mean score for both groups was 72.48±1.05 (95% CI=70.4–74.5); 86.2±0.96 (95% CI=84.2–88.0); and 77.7±0.72 (95% CI=76.3–79.1), in the pre-test, the simulation and the post-test, respectively. The students of group A self-evaluated their performance with 3.93/5.00, and those of Group B, 4.04/5.00.
DiscussionThis study showed that Group A students did not score lower on simulation-based activity when compared to students in Group B, suggesting that before the fifth year of undergraduate medical education, students could properly develop an informed consent-process for health research if they receive early education about medical ethics and research bioethics. Issues related to bioethics in human health research can be included as soon as medical students initiate research methodology courses.
La capacitación mediante la simulación médica permite un aprendizaje continuo bajo condiciones controladas. Las actividades de capacitación basadas en simulación se pueden utilizar simultáneamente con otras estrategias educativas para fortalecer las habilidades actitudinales que se necesitan para desarrollar un proceso de consentimiento informado en el contexto de la investigación en salud.
ObjetivoFacilitar un aprendizaje en estudiantes de medicina de pregrado y evaluar sus competencias para llevar a cabo un proceso de consentimiento informado en un escenario que semeje la realidad.
Materiales y métodosEn este estudio semilongitudinal desarrollamos una actividad basada en simulación con 136 estudiantes de medicina de cuarto (grupo A) y quinto año (grupo B) de la Universidad Marista de Mérida, al sur de México.
ResultadosEl puntaje promedio para ambos grupos fue 72,48±1,05 (IC 95%=70,4-74,5); 86,2±0,96 (IC 95%=84,2-88,0), y 77,7±0 72 (IC 95%=76,3-79,1) en el pre-test, la simulación y el pos-test, respectivamente. Los estudiantes del grupo A se autoevaluaron con 3,93/5,00 y los del grupo B, 4,04/5,00.
DiscusiónNuestro estudio mostró que los estudiantes del grupo A no tenían puntajes más bajos en la actividad basada en simulación al compararlos con los estudiantes en el grupo B, sugiriendo que antes del quinto año de educación médica de pregrado los estudiantes podrían desarrollar adecuadamente un proceso de consentimiento siempre que reciban educación temprana sobre ética médica y bioética de investigación. Los temas relacionados con la bioética en la investigación en salud humana pueden incluirse tan pronto como los estudiantes de medicina inician los cursos de metodología de la investigación.
High fidelity simulation training involves learning under specific circumstances in an environment that depict real life situations. Simulation training allows learning in a controlled, but also accurate, realistic, safe and secure environment. Simulation can be used for clinical skill development, but also for building attitudinal competences among medical students.1,2
Competences for observational, epidemiological, biomedical or clinical research are important for medical doctors that work at hospitals, clinics, research centers or academic settings, but health research involving patients must be conducted strictly adhered to the bioethical principles that establish the respect of human subjects, including the respect for their autonomy, non-maleficence, beneficence and justice.3 These basic principles are taught in several medical undergraduate academic programs,4 but it is the application of those principles that allow the comprehension of their relevance by young medical doctors.
Previous studies have shown that there is a need to implement measures to improve the adherence of research to bioethical principles, including the training for medical researchers to help patients to reasonably understand during informed consent process. Recent studies report that methodological issues of medical research such as randomization, voluntariness, right to withdraw, risks or benefits of the studies, are only understood by a 44–57% of participant in research trials. Commonly, if not well informed, patients might misconceive regular medical treatment with research trials.5
Adherence to bioethical principles application is important when it comes to conduct health research in developing countries such as Mexico, where the literacy and academic level of underserved population require an effort from health researchers and medical doctors to accurately provide the precise information to their patients to help a full understanding the objectives, alternatives and potential consequences of participating in research studies.6,7
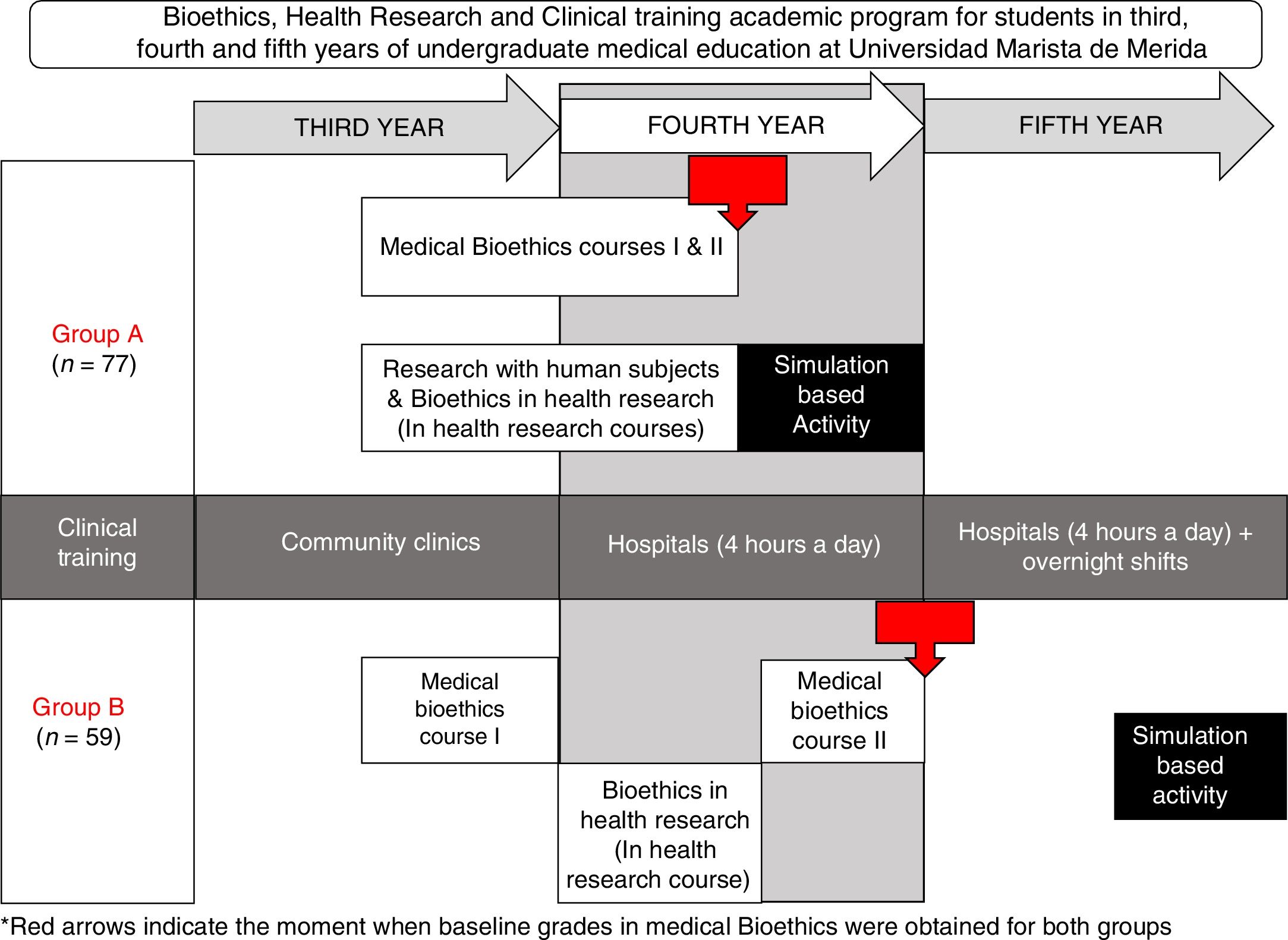
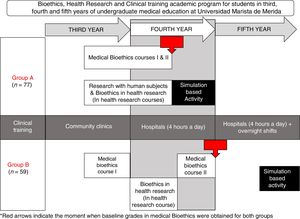
To facilitate a significant learning in our undergraduate medical students and evaluate their competences to conduct an informed consent process in a controlled real-life scenario, we developed a simulation-based activity in two groups of medical students. There were certain differences between the two groups: in Group A, students were younger, and they also had less experience in working with hospitalized patients when compared to group B; but another difference was that Group A had an earlier introduction to medical ethics and bioethics in health research (Fig. 1).
With the antecedents of an earlier introduction to bioethics in group A, and the previous semester grade reports in medical ethics, we hypothesized that group A would not have significantly lower achievement in the simulation-based activity, when compared to group B.
If our hypothesis was true, it would mean that we can successfully train our students to conduct informed consent process adequately before that end the fourth year of medical education, just before they intensify their clinical practices at the hospitals.
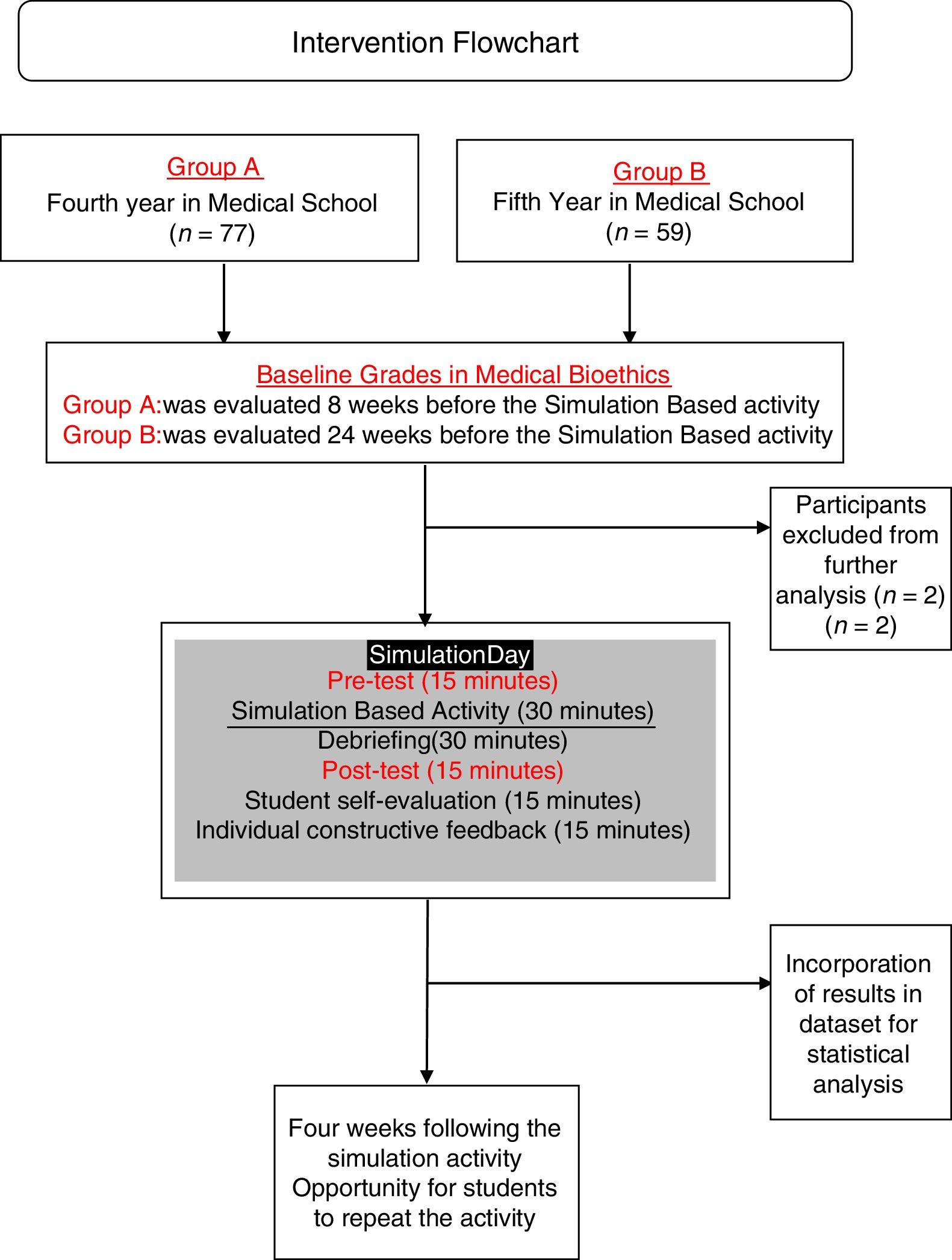
Materials and methodsIn this semi-longitudinal study, we developed a simulation-based activity with students from years four and five of the medical doctor undergraduate academic program at Universidad Marista de Merida in Southern Mexico.
Learning activitiesWe worked with two groups of undergraduate medical students, Group A and Group B, both groups had two previous medical bioethics courses, bioethics in health research and research with human subjects.
As the medical undergraduate academic program at Universidad Marista de Merida includes an early involvement of students in health research, we modified the timing of the bioethics in health research topics to allow a simultaneous learning of both, bioethics in health research and research involving human subjects.
Research with human subjects was only provided to group A and consisted of the historic background, from medical ethics deontology to bioethics of the XX century, the origins of ethical dilemmas in that period.
For both groups, bioethics in health research involved the following learning activities: (a) Four hours of independent study, (b) twelve hours of online certifications in Health research involving human subjects and Good Clinical Practices, (c) four hours of lectures, two per year, (d) two three-hour sessions of case reviews and problem based research bioethics analysis, one session of observational studies and one session of bioethics in experimental studies with emphasis on clinical trials. The Simulation-Based Activity consisted of a thirty-minute activity at the Montagne Medical Simulation Center, where the student performed individually the informed consent process based on a randomly assigned case of the author's’ design.
The learning evaluations for Group A and Group B during their previous medical ethics course consisted of a case-specific student-designed informed consent form evaluation and a written test. Medical Ethics grades averaged the informed consent form with a written evaluation.
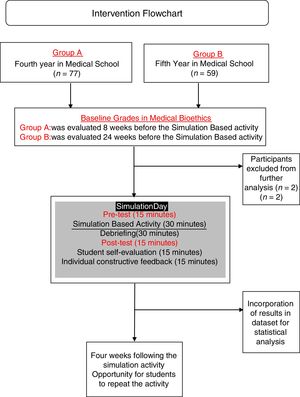
Learning evaluationFor Group A and B, evaluations included the certificates of completion, group clinical-case analysis and case-based multiple option test before and after the activity at the simulation center. Cases chosen for the activity were chosen and edited by authors from real-life cases open access repositories and adapted (epidemiologically, ethnically and culturally) to the local context of the population living in Southern Mexico and the checklist was designed also by authors and validated for clarity and construct validity by experts from the university's department of academic innovation. The simulation activity was evaluated using a 22-item validated checklist (Appendix, supplementary material) followed by debriefing sessions. Simulation activity was evaluated by trained medical interns and debriefing sessions were conducted by interns and the professor. The pre-test and post-test feedback was provided together after the post-test and simulation-activity evaluation. (Fig. 2) Students received different cases for the pre-test and for post-tests.
The simulation-based activity was anonymously evaluated by the students. Students were also asked to express (a) their self-perceived performance (scale 1–5); (b) their opinion about the relevance of the simulation activity (scale 1–5); (c) previous participation in health research projects and (d) if they perceive that their oral communication skills influenced on their performance during the simulation-based activity, and if so, if it influenced in a positive or negative way. Students who obtained <60% points repeated the simulation within a four-week period, as many times as needed. All other students could repeat the simulation practice to improve their proficiency.
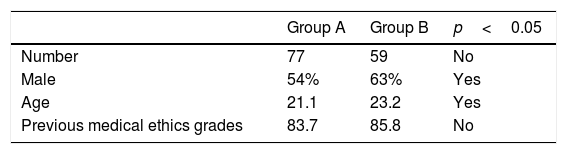
ResultsA total of 136 students from GROUP A and GROUP B were initially included for the present study. Two students were excluded from analysis because they were retaking the course. Baseline characteristics of both groups are shown in Table 1.
Pre-test average scores were 72.48±1.05 (Confidence Interval 95%=70.4–74.5); while simulation-based activity average score was 86.2±0.96 (Confidence Interval 95%=84.2–88.0) and the post-test was 77.7±0.72 (Confidence Intervals 95% 76.3–79.1).
Eighteen (23%) of the students of GROUP A reported antecedents of participating in health research, but the average simulation scores did not differ significantly. Of the 59 students in GROUP B, 14 (24.0%) reported to have previously participated in health research projects (87.3). Students without previous experience in health research had significantly lower average simulation activity score points (81.2; p=0.028) when compared using a two-group mean comparison test (t-test).
Average score points in the simulation activity of the students who perceived that their communication skills influenced on their performance, did not really differ significantly even when they perceived their skills as positive.
For both groups, the average perceived relevance of the simulation-based activity was 4.78±0.04 (Confidence Intervals 95%=4.68–4.86); 4.67 for GROUP B and 4.85 for GROUP A; and even when perceived relevance differed slightly, difference was not significant.
Students from GROUP A self-rated their performance in the simulation-based activity with a mean score of 3.93 out of 5.00; while GROUP B mean self-score was 4.04; showing no difference in perceived performance between groups.
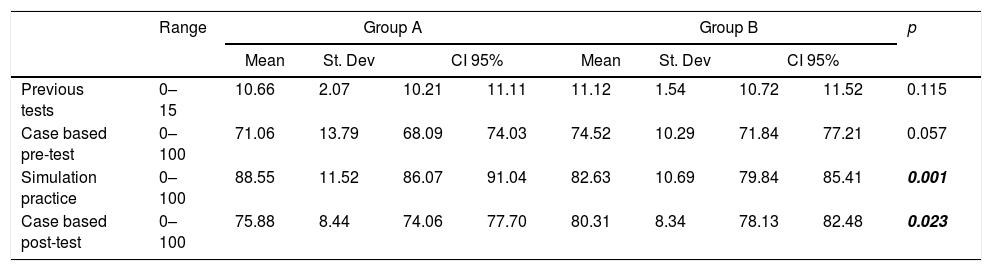
GROUP A obtained higher average point score in the simulation-based activity, while Group B performed better in case-based post-tests (p<0.05 in both cases). Both groups increased their point average from pre-test to post-test significantly (p<0.01), GROUP A from 74.5±to 80.3, and GROUP B increased from 71.0±1.5 to 75.8±0.91 (Table 2).
Student performance for Groups A and B.
| Range | Group A | Group B | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | St. Dev | CI 95% | Mean | St. Dev | CI 95% | |||||
| Previous tests | 0–15 | 10.66 | 2.07 | 10.21 | 11.11 | 11.12 | 1.54 | 10.72 | 11.52 | 0.115 |
| Case based pre-test | 0–100 | 71.06 | 13.79 | 68.09 | 74.03 | 74.52 | 10.29 | 71.84 | 77.21 | 0.057 |
| Simulation practice | 0–100 | 88.55 | 11.52 | 86.07 | 91.04 | 82.63 | 10.69 | 79.84 | 85.41 | 0.001 |
| Case based post-test | 0–100 | 75.88 | 8.44 | 74.06 | 77.70 | 80.31 | 8.34 | 78.13 | 82.48 | 0.023 |
St. dev, standard deviation; CI 95%, confidence interval 95%. Significant p values are shown in bold, italic font.
While analyzing the results from the simulation learning practice checklist item by item, we found no significant differences, except for two items: that students from GROUP B were more prone to use technical terminology without describing the meaning of the medical terms to the patient or family.
The students’ self-perceptions about their performance were commented on their own words during debriefing. Students from both groups perceived that they lacked order, that they could have performed better with a structured speech to not forget any aspect. Students in GROUP A mainly forgot to provide important information, while Group B were more prone to forget to give the patient a chance to ask them or clarify specific details of the research.
DiscussionWe have described the learning activities linked to bioethics in health research involving human subjects and how two generations of undergraduate students performed in them. Previous educational researches have included educational ethical issues in simulation learning practices; Gisondi and colleagues developed an activity where they analyzed the observance of bioethical principles during critical care, including informed consent, finding that under stressful situations where the life of patients is compromised, ethical considerations may be missed.8 But when informed consent is not related with therapeutic emergency medicine, but research purposes, informed consent process takes place in a different situation. In Latin America ethical misconduct in conducting health research has been reported, most of the patients enrolled in clinical trials belong to underserved population commonly are unaware of their participation in a trial and are recruited by their physicians to access the treatment they need.9
This situation of lack of compliance with bioethical principles has been reported in Mexico, where a study reported that principal investigators of medical trials did not provide truthful, coherent information about who obtained consent forms for their studies; only 34% of principal investigators reported to have previous training in medical ethics. Two thirds of the principal investigators gave only a few minutes for the patient to decide if they would participate or not in the trial; they also stated how they were unsure of how and where the informed consent forms were kept, while only 16% gave a copy of the informed consent form to the participant. Finally, 67% of the principal investigators considered that the patients understood reasonably the information they provided.10
In our study, we replicated different cases in which the student was asked to develop the informed consent process, facilitating the patientś comprehension and our results showed that students that are closer to begin their hospital rotatory internship are more prone to use medical terms without explaining their meaning to the patients, even when they were asked to facilitate the patientś understanding, and obtained an overall lower point score in the simulation learning practice. We hypothesize that these differences could be related to a previously described phenomenon in medical education: the gradual decrease of empathy. Diseker and Michielutte, identified decades ago a decrease of empathy among medical students, particularly prior to and following clinical experiences, which might be consistent with our findings with Group A students. A lack of empathy could be prevented in future generations, if as reported by Hojat et al., empathy is an amendable issue among undergraduate medical students.11–13
To achieve adherence to the bioethical basic principle of voluntary participation in health research involving human subjects, the informed consent process should go beyond a simple presentation of the informed consent form, it should involve a good communication between researcher and patient.14 For that reason, a good performance in the simulation-based activity required more than to just reading a contract, it required the integration of knowledge, problem solving skills, empathy and communication skills. In our study, we found no significant data regarding perceived oral communication skills and the performance in the simulation learning practice.
We found that students from GROUP B who had previous experience in health research performed better during the simulation activity, and we consider that a possible explanation that point scores in the simulation-based activity of students from GROUP A did not vary by previous experience in health research is that medical students who participate in research studies are not commonly in direct communication with the patients. In a study conducted by Nikkar-Esfahani and colleagues, where the roles of graduate medical students in research projects were reported, it called our attention that the students did not have direct contact with patients, on the contrary, at our university, undergraduate medical students are gradually involved in health research so even when they do not have contact with patients initially, they eventually do, commonly before their last year of undergraduate education.15
Our study showed that students from GROUP A did not have significantly lower average point scores in the simulation-based activity than students in GROUP B. GROUP A had significantly higher scores in the simulation learning practice, but also lower scores in post-test. More studies and prospective experiences could help understand the underlying reasons for these differences, but we know that study habits and attitudes toward evaluation change between generations and might be related to the differences regarding written tests or simulation-based activities. Another aspect that should be taken in account is that students in GROUP A are at the stage of having continuous practices at the simulation center, while students in GROUP B have more practices at clinical settings than at the simulation center, for that reason, we cannot discard the possibility that students in GROUP A might feel more comfortable at the simulation center than GROUP B.16
In conclusion, we accept our hypothesis that medical students in their fourth year of undergraduate education performed equally the conduction of a simulated informed consent process, when compared to their fifth-year peers. Younger students had significantly higher score points in the simulation learning practice, but lower score points in case-based pre-tests and post-test, but in both evaluations, the simulation-based activity had a positive impact on the students’ case-based tests.
Themes regarding bioethics in health research involving human subjects, including simulation for developing informed consent process can be included as early as undergraduate medical students start health research methodology courses.
Ethical disclosuresThe authors declare that they have followed their institution's protocols on the publication of participant data and that all participants included in the study have received enough information and have given their written informed consent to participate in this study.
Conflict of interestThe authors have no conflicts of interest to declare.
All authors thank Joeanna Cambranis for her invaluable, continued technical assistance. Second author thanks for the Mexican Council of Science and Technology (Conacyt) for his postdoctoral scholarship.