P-38 - UNVEILING THE IMPACT ON IN UTERO EXPOSURE TO BIOLOGIC TREATMENTS FOR INFLAMMATORY BOWEL DISEASE (IBD) ON CHILDREN'S PSYCHOMOTOR DEVELOPMENT: INSIGHTS FROM THE DUMBO REGISTRY OF GETECCU
1Hospital Infantil Universitario Niño Jesús, Gastroenterology Unit, Madrid. 2Hospital Infantil Universitario Niño Jesús Gastroenterology Unit, Madrid. 3Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa IIS-IP and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas CIBEREHD, Gastroenterology Unit, Madrid. 4Hospital Universitario Virgen del Rocío, Gastroenterology Unit, Sevilla. 5Complexo Hospitalario Universitario de Santiago de Compostela, Gastroenterology Unit, Santiago de Compostela. 6Hospital Universitario Marqués de Valdecilla e IDIVAL, Gastroenterology Unit, Santander. 7Hospital Universitario Puerta de Hierro, Gastroenterology Unit, Majadahonda. 8Hospital Clínico Universitario Lozano Blesa, IIS Aragón y CIBERehd, Gastroenterology Unit, Zaragoza. 9Hospital Clinic de Barcelona, Centro de Investigación Biomédica en Red Enfermedades Hepáticas y Digestivas CIBERehd, Institut d'Investigacions Biomèdiques August Pi i Sunyer IDIBAPS, Gastroenterology Unit, Barcelona. 10Hospital Universitario Central de Asturias, Diet- Microbiota and Health Group, Instituto de Investigación Sanitaria del Principado de Asturias ISPA, Gastroenterology Unit, Oviedo. 11Hospital Universitario 12 de Octubre, Gastroenterology Unit, Madrid. 12Xerencia Xestión Integrada de Vigo- SERGAS, Grupo de Investigación de Patología Digestiva, Instituto de Investigación Sanitaria Galicia Sur IIS Galicia Sur, SERGAS UVIGO, Gastroenterology Unit, Vigo. 13Hospital Universitario de Bellvitge-L'Hospitalet de Llobregat, Gastroenterology Unit, Barcelona. 14Hospital Universitario Fundación Alcorcón, Gastroenterology Unit, Madrid. 15Complexo Hospitalario Universitario de Ourense, Gastroenterology Unit, Ourense. 16Hospital Universitario de Galdakao, Biobizkaia Health Research Institute, Gastroenterology Unit, Bizkaia. 17Hospital Universitario Miguel Servet, Gastroenterology Unit, Zaragoza. 18Hospital Universitario de Cabueñes, Gastroenterology Unit, Gijón. 19Hospital Universitario de Fuenlabrada, Gastroenterology Unit, Madrid. 20Hospital Universitario de Burgos, Gastroenterology Unit, Burgos. 21Complejo Hospitalario Universitario de Ferrol, Gastroenterology Unit, A Coruña. 22Hospital General Universitario de Ciudad Real, Gastroenterology Unit, Ciudad Real. 23Hospital Trueta de Girona, Gastroenterology Unit, Girona. 24Hospital General Universitario Dr. Balmis Alicante, ISABIAL-CIBERehd, Gastroenterology Unit, Alicante. 25Hospital Universitario de Torrejón- Universidad Francisco de Vitoria, Gastroenterology Unit, Madrid. 26Hospital Universitario Joan XXIII, Gastroenterology Unit, Tarragona. 27Hospital Universitario Juan Ramón Jiménez, Gastroenterology Unit, Huelva. 28Consorci Sanitari de Terrassa CST, Gastroenterology Unit, Barcelona. 29Hospital General de Tomelloso, Instituto de Investigación Sanitaria La Princesa- Instituto de Investigación Sanitaria de Castilla-La Mancha IDISCAM- Centro de Investigación Biomédica en Red Enfermedades Hepáticas y Digestivas CIBERehd, Gastroenterology Unit, Ciudad Real. 30Hospital Universitario La Paz, Gastroenterology Unit, Madrid. 31Hospital Universitario de Álava, Gastroenterology Unit, Álava. 32Hospital Clínico Universitario San Cecilio, Gastroenterology Unit, Granada. 33Hospital Vall d´Hebron, Gastroenterology Unit, Barcelona. 34Hospital Universitario Reina Sofía, Gastroenterology Unit, Córdoba. 35Hospital Universitario Virgen de la Victoria, Gastroenterology Unit, Málaga. 36Hospital Universitario San Agustín, Gastroenterology Unit, Avilés. 37Hospital Universitario Nuestra Señora de Candelaria, Gastroenterology Unit, Tenerife. 38Hospital Mancha Centro, Gastroenterology Unit, Álcazar de San Juan. 39Hospital Universitario del Henares, Gastroenterology Unit, Coslada. 40Hospital General Universitario de Valencia, Gastroenterology Unit, Valencia. 41Hospital del Mar, IMIM (Hospital del Mar Medical Research Institute), Gastroenterology Unit, Barcelona. 42Hospital Universitario Son Espases, Gastroenterology Unit, Palma de Mallorca. 43Bioodonostia Health Research Institute - Donostia University Hospital, Universidad del País Vasco (UPV/EHU), Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Gastroenterology Unit, San Sebastián. 44Hospital Universitario Rey Juan Carlos, Gastroenterology Unit, Móstoles. 45Hospital Sant Joan de Déu, Manresa; Hospital Universitario de La Princesa and CIBEREHD, Madrid. 46Hospital Santos Reyes Aranda de Duero SACyL, Gastroenterology Unit, Burgos. 47Hospital Universitario de Canarias, Gastroenterology Unit, La Laguna. 48Hospital Clínico Universitario de Valencia, Gastroenterology Unit, Valencia. 49Hospital Universitario Infanta Sofía, Gastroenterology Unit, San Sebastián de los Reyes. 50ISADMU Centro Médico Teknon, Gastroenterology Unit, Barcelona. 51Hospital de Granollers, Gastroenterology Unit, Barcelona. 52Hospital Infantil Universitario Niño Jesús, Intensive Care Unit, Madrid.
Introduction: Our aim was to evaluate the impact of the exposure of biologics in utero on the psychomotor development of children during the first year of life.
Methods: Data from children included in the DUMBO registry with complete AQS-3 available up to 12 months of age were analysed. DUMBO is a prospective, observational, and multicentre registry endorsed by GETECCU, which enrolls pregnant women with IBD throughout 5 years in 70 centres in Spain. Study protocol is summarized in figure 1a. Normal psychomotor development was defined by ASQ-3 scores above the lower limit of normality (referral zone) in all domains. Serious adverse events (SAE) were defined in accordance with the ICH Topic E 2 A Clinical Safety Data Management.
Results: 352 children born to 343 mothers were included (9 pair of twins) (tables 1a, 1b and 1c). 134 children (38%) had been exposed to biologics in utero; from them, 50 (37%) had been exposed to adalimumab, 44 (32%) to infliximab, 3 (2.2%) to certolizumab, 1 (0.7%) to golimumab, 28 (20%) to ustekinumab, and 10 (7.5%) to vedolizumab. 8% of the mothers were smokers during pregnancy; no other toxic consumption (alcohol or drugs) was recorded. The ASQ-3 results across different domains are presented in figure 1b, and the impact of the different factors associated with the neurodevelopment is summarised in table 1d. In the multivariate analysis, to have been born to a mother with CD (vs. UC) was associated with higher likelihood (OR = 2, 95%CI = 1.1-3.9), while to be premature was associated with lower likelihood (OR = 0.3, 95%CI = 0.1-0.6) of having ASQ-3 scores above the limit of normality in all domains at 12 months of age.
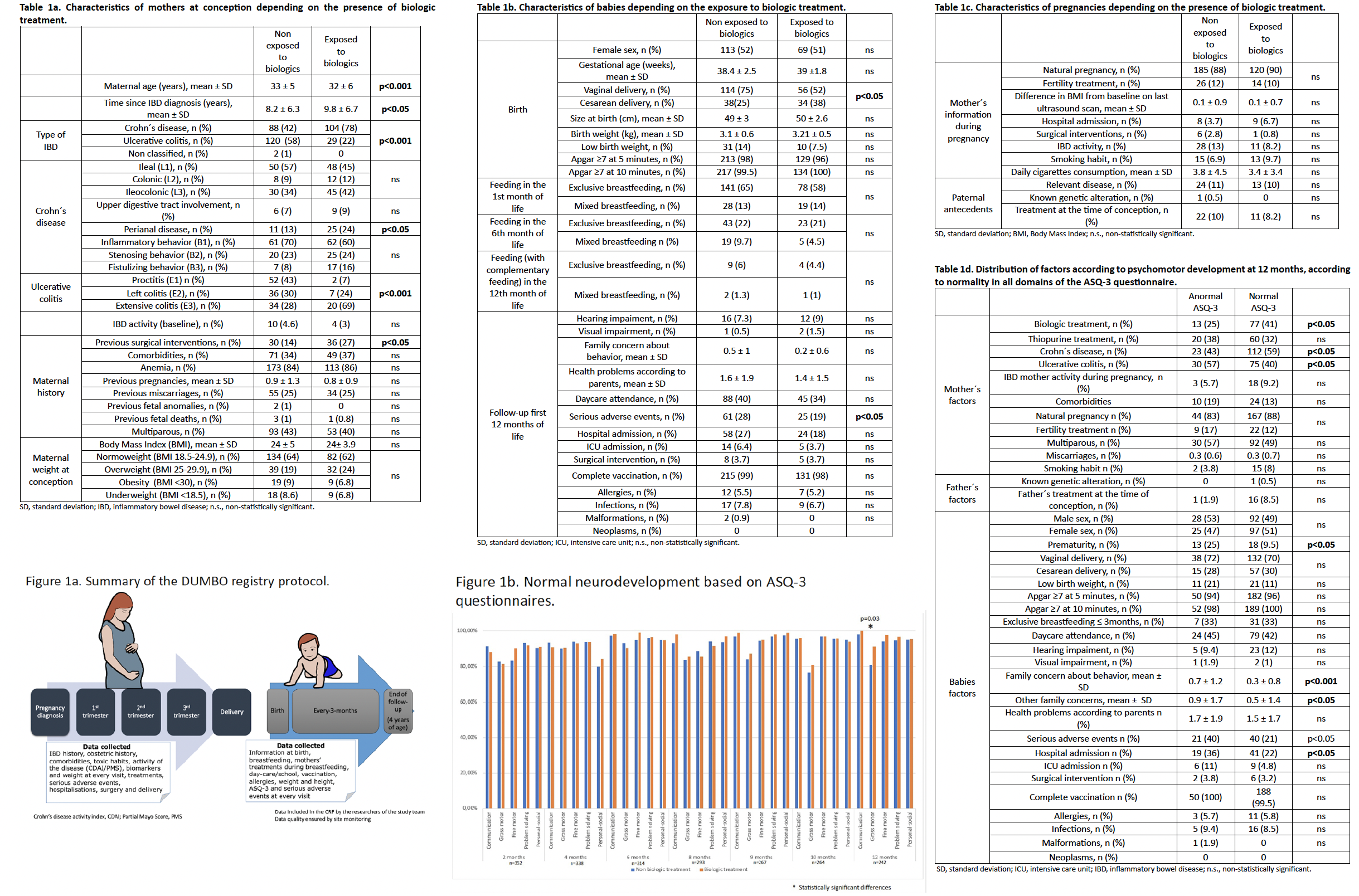
Conclusions: In the multicenter, prospective DUMBO registry, the exposure to biologics for the treatment of IBD in utero (including anti-TNF and non-anti-TNF agents) did not impair the psychomotor development of the children.









