49 - CLINICAL AND ENDOSCOPIC RESPONSE TO TREAT-TO- TARGET VERSUS STANDARD OF CARE IN CROHN'S DISEASE PATIENTS TREATED WITH USTEKINUMAB: WEEK 48 RESULTS OF THE STARDUST TRIAL
1Department of Gastroenterology, Humanitas University, Milan (Italy). 2University Hospitals Leuven, Leuven (Belgium). 3Academic Medical Centre, University of Amsterdam (The Netherlands). 4Department of Gastroenterology, Hospital Clinic of Barcelona, CIBERehd, Barcelona. 5Department of Medicine I, Agaplesion Markus Hospital, Frankfurt/Main (Germany). 6Department of Pharmacology & Therapeutics, Institute for Molecular and Cell Biology, Faculty of Medicine University of Porto and Department of Gastroenterology, Hospital de Sao Joao, Porto (Portugal). 7Janssen-Cilag Polska Sp. zoo., Warsaw (Poland). 8Janssen-Cilag, Issy-les-Moulineaux (France). 9Janssen-Cilag BV, Breda (The Netherlands). 10Janssen-Cilag Russia, Moscow (Russian Federation). 11Janssen-Cilag S.p.A., Milan (Italy). 12Janssen Global Services, LLC, Horsham, PA, (USA, at the time of study). 13Gastroenterology Unit, Mauriziano Hospital, Turin (Italy). 14Clinical and Research Center for Inflammatory Bowel Diseases, Clinical Center ISCARE, Prague (Czech Republic). 15IBD Unit, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome (Italy). 16Department of Gastroenterology, Glasgow Royal Infirmary, Glasgow (United Kingdom). 17INSERM Unité 954 and Department of Hepato-Gastroenterology, University Hospital of Nancy, University of Lorraine, Hudemont (France).
Introduction: STARDUST, a phase 3b randomized trial, compared treat-to-target (T2T) maintenance strategy vs standard of care (SoC) in Crohn’s disease (CD) patients (pts) treated with ustekinumab (UST). Here we describe Week (W) 48 endoscopic (1o endpoint) and clinical results.
Methods: Adults with moderate–severely active CD ([CDAI] 220-450), [SES-CD] ≥ 3); received IV UST ∼6 mg/kg at W0 (baseline [BL]); SC UST 90 mg at W8. At W16, CDAI 70 responders were randomized 1:1 to T2T or SoC. T2T pts received SC UST q12w/q8w based on 25% improvement in SES-CD score vs BL and intensified to q4w from W16–48 if failure to meet: CDAI 70 point improvement, and CRP ≤ 10 mg/L or faecal calprotectin (FCal) # 50% reduction in SES-CD score vs BL).
Results: Of 500 pts, 441 achieved CDAI 70 response at W16 and were randomized to T2T (n = 220, 79.1%) or SoC (n = 221. 87.3%); and completed W48. More pts in T2T (37.7%) vs SoC (29.9%) achieved the 1o endpoint at W48: (p = 0.0933; NRI, non-responder imputation). At W48, clinical response rates were T2T 68.2% and SoC 77.8% (p = 0.0212; NRI); clinical remission 61.4% vs 69.7% (NS;NRI), respectively; ≥ 50% improvement in FCal 39.4% vs 46.5% (NS;NRI)/63.1% vs 60.6% (NS; LOCF, last observation carried forward) and CRP 41.7% vs 53.3% (p = 0.032; NRI)/53.2% vs 57.2% levels (NS; LOCF), (Table). In T2T and SoC, 59.2% (122/206) and 53.2% (116/218) of pts started on UST q12w; of those, 59.8% (73/122) and 63.8% (74/116) were still on q12w at W48. Of pts who started on q8w, T2T, 40.5% (34/84) and SoC 78.4% (80/102) remained on q8w at W48. At W48, 17% (35/206) of pts were on UST q4w in T2T. No new safety signals were reported.
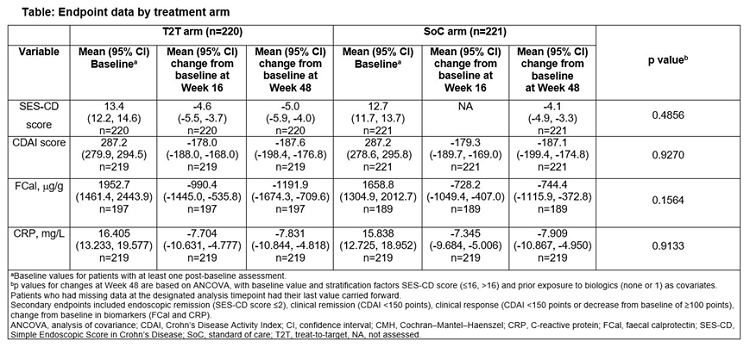
Conclusions: STARDUST is the first trial to use endoscopy to guide dose escalation in pts with CD. After 48W of maintenance therapy with UST, more pts achieved the endoscopic response with T2T vs SoC.









