82 - INFLAMMATORY BOWEL DISEASE NEW-ONSET DURING SECUKINUMAB THERAPY: REAL-WORLD DATA FROM A TERTIARY CENTRE
1Aparato Digestivo; 2Unidad EEI, Aparato Digestivo, Hospital General Universitario de Elche 3Estudiante Grado en Medicina, Universidad Miguel Hernández, Elche. 4Unidad de Farmacia Hospitalaria; 5Radiodiagnóstico, Hospital General Universitario de Elche.
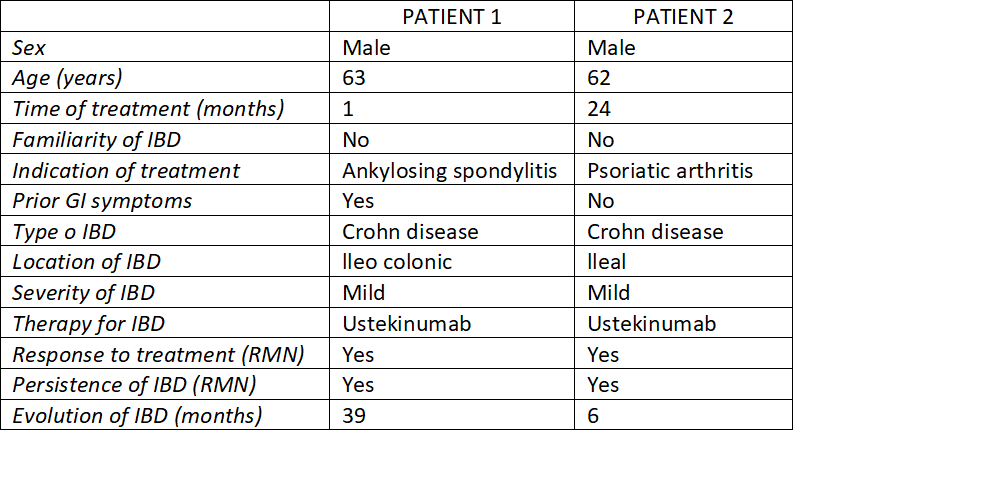
It is well known that the spectrum of inflammatory bowel disease (IBD) involves rheumatologic and dermatologic disorders by sharing common inflammatory and genetic pathways. Secukinumab, an interleukin - 17A inhibitor, is currently prescribed for the treatment of psoriasis, psoriatic arthritis and ankylosing spondylitis. Even though this treatment is regarded as safe for patients, during the last years several cases of new- onset IBD have been reported in the literature in patients receiving secukinumab, with an incidence of 1%. We carried out a descriptive, retrospective study by means of checking out the patients treated with secukinumab for dermatologic and rheumatologic disorders from January 2017 to December 2020 in our hospital. We selected those who developed an IBD after the beginning of the treatment in order to estimate the cumulative incidence in this population. Of the overall 127 patients receiving secukinumab, two cases of new-onset Crohn´s disease were identified in our center, which corresponds to a cumulative incidence of 1.5%. Patients had ankylosing spondylitis and psoriatic arthritis. The time to onset was from 1 to 24 months of secukinumab exposure and the IBD was confirmed by magnetic resonance and afterwards ileocolonoscopy and selected biopsy. The two patients (100%) presented persistent lesions in the terminal ileum 6 months after discontinuing secukinumab and despite IBD therapy with corticosteroids and ustekinumab. While clinical trials have revealed clusters of new IBD cases amongst patients using an IL-17 inhibitor, there remains a lack of evidence to suggest a causal relationship. In our center we identified 2 patients with new- onset Crohn´s disease after secukinumab treatment (1.5%). One of the patients had previous gastrointestinal symptoms (diarrhea) and no one told familiar history of IBD. Careful clinical investigation of patients with respect to possible susceptibility to IBD prior to secukinumab therapy is strongly advised.